
Astonishing Video Shows Hydrogen And Oxygen Forming Water At The Nano Scale
Scientists Capture the Smallest Water Bubble Ever Seen and It Could Change Space Travel Forever
The formation of water is something that often goes unnoticed in our daily lives. Whether it’s forming in our kitchens, dripping on our windows during rainy days, or collecting in our gardens, it’s an event we take for granted. However, in 2024, scientists at Northwestern University observed something truly extraordinary: water being formed from hydrogen and oxygen molecules on a scale so minuscule that it challenges everything we know about chemistry at the atomic level. This breakthrough not only pushes the boundaries of science but also promises to revolutionize future space missions and provide new solutions for long-term survival in space.
A Glimpse into the Limits of Nature’s Chemistry
This discovery is not merely a scientific triumph, but a window into understanding chemistry at its most fundamental scale. The implications of this achievement extend far beyond the laboratory. The ability to observe water formation at the molecular level opens up new possibilities for sustainable resource generation in space and deepens our understanding of catalytic reactions on Earth. As researchers continue to examine these minute chemical processes, we are forced to rethink what is possible when science operates at the nanoscale.
How Scientists Watched Water Form One Molecule at a Time
The breakthrough in observing water formation at this incredibly small scale was the result of years of effort and innovation. One of the main challenges was developing a way to stabilize the reactive gases long enough for scientists to capture the process visually. To achieve this, the team designed honeycomb-shaped nanoreactors made of ultra-thin glassy membranes. These structures allowed hydrogen and oxygen molecules to be confined in controlled spaces, providing an ideal environment for high-vacuum transmission electron microscopy, an advanced imaging technique. The unusual combination of stability and transparency of these nanoreactors allowed researchers to see reactions in real time that had previously been too fast or unpredictable to capture.
By incorporating palladium into these nanoreactors, the team was able to observe how the metal interacted with incoming hydrogen at a level of detail that traditional imaging methods could not match. This novel approach allowed for not only direct observation but also a detailed tracking of the structural changes within the palladium as it absorbed hydrogen. This real-time tracking of the reaction process was crucial to understanding how water begins to form under such precise conditions.
This research exemplifies the power of combining advanced imaging techniques with meticulously engineered nanoscale environments. It also underscores the significance of designing targeted instrumentation that can turn abstract chemical models into tangible, visible phenomena. The breakthrough was a perfect example of how scientific innovation can shed light on previously hidden processes.
The Smallest Water Bubble Ever Recorded
When the scientists witnessed the formation of a nanosized water bubble, they were astounded. As Liu, one of the lead researchers, remarked, “We think it might be the smallest bubble ever formed that has been viewed directly. It’s not what we were expecting. Luckily, we were recording it, so we could prove to other people that we weren’t crazy.” In that moment, what had long been regarded as an inferred event in textbooks became something concrete and visible: a tiny droplet of water forming within a metal framework.
At such a small scale, describing something as a "bubble" means identifying a distinct region where water accumulates and separates from its surroundings. This was not just a vague hint of moisture or scattered molecules; it was an actual formation of liquid water. The team could observe this process as it unfolded inside the palladium, providing an unprecedented glimpse into the behavior of water in environments much smaller than a typical cell.
To validate their findings, the researchers employed electron energy loss spectroscopy (EELS), a technique often used in space exploration, including aboard India’s Chandrayaan 1 mission to confirm the presence of water on the Moon. EELS analyzes how electrons lose energy as they pass through a material, and this method revealed the characteristic signatures of water molecules in the bubble. This dual approach of visual observation coupled with spectroscopic confirmation not only verified that the bubble was indeed water but also offered insights into the conditions that enable water to form rapidly under such unique circumstances.
Implications for Future Space Missions
While the scientific achievement is remarkable on its own, its real-world applications, particularly for space exploration, are truly groundbreaking. As Vinayak Dravid, the study’s senior author, pointed out, this discovery has significant implications for future space missions. He drew a parallel to the movie The Martian, where the character Mark Watney uses a rocket fuel-based process to extract hydrogen and combine it with oxygen to produce water. Dravid explained, “Our process is analogous, except we bypass the need for fire and other extreme conditions. We simply mixed palladium and gases together.”
In long-term space missions, water becomes one of the most vital resources. Due to the constraints of carrying enough water to sustain astronauts on extended journeys, it’s often impractical to bring enough supplies from Earth. Additionally, extracting water from local sources, such as ice deposits on celestial bodies, is highly dependent on landing sites and technology. Therefore, the ability to generate water from hydrogen and oxygen—two substances that are readily available on spacecraft—could be a game-changer for deep space exploration.
Palladium’s Role in Revolutionizing Water Systems
A key finding of the study was the role palladium plays in water generation. The researchers discovered that the sequence in which hydrogen and oxygen are introduced affects the rate at which water forms. Palladium, which is a metal known for its catalytic properties, responds differently depending on which gas is introduced first. This insight could inform the design of future catalytic systems, making the process of water generation more efficient and tailored to specific needs in space.
If palladium can be pre-saturated with hydrogen, astronauts might only need to add oxygen to generate drinkable water—a simple yet effective solution for a critical need in space missions. This discovery could pave the way for deeper innovations in life-support systems for space exploration. The potential applications extend beyond space: similar catalytic systems might be used on Earth to improve hydrogen-based energy technologies and other industrial processes.
A Deeper Understanding of Nature’s Smallest Processes
This groundbreaking research, published in Proceedings of the National Academy of Sciences, marks a significant leap in our ability to observe and understand chemical reactions at the atomic scale. For decades, scientists could only speculate about what occurs at this scale. Now, thanks to advanced imaging and spectroscopy, they can directly observe these fundamental processes, which are essential to understanding everything from how metals store hydrogen to how catalysts accelerate chemical reactions.
By observing these reactions in real time, scientists are gaining insights into natural processes that begin at a level too small for the human eye to see. These findings could inform the development of smarter, more efficient technologies, especially those that aim to harness natural processes in sustainable and environmentally friendly ways.
A Broader Impact: Micro Water Dynamics in Ecosystems
While this study focuses on nanoscale chemistry, its findings have the potential to influence how scientists approach the study of water in natural ecosystems. Birds and other wildlife often rely on small, transient water sources, such as moisture trapped in soil, on leaves, or in small puddles. These microhabitats can change rapidly and are difficult to measure with traditional tools. By applying the techniques developed in this study, scientists could improve their ability to detect and understand how small water sources support animal behaviors, migration patterns, and overall ecosystem health.
Although the lab findings don’t directly replicate natural settings, they offer valuable insights into how water can form and behave in confined spaces. As climate change and environmental degradation continue to affect ecosystems, understanding the behavior of water at every scale—even at the nanoscale—becomes increasingly important for conservation efforts.
Conclusion: The Significance of Small Moments
At first glance, the creation of a nanosized water bubble might seem insignificant. However, this research holds a deeper lesson: many of the most important discoveries in science come from observing the smallest, most subtle phenomena. Just as water shapes landscapes and hydrogen fuels stars, this tiny bubble could potentially shape the future of space exploration. By looking closely and questioning the seemingly inconsequential, we open the door to breakthroughs that can transform our understanding of the universe.
As we continue to explore space, discoveries like these remind us that even the smallest moments can have profound implications for the future.
Sources:
-
Proceedings of the National Academy of Sciences
-
NASA's Chandrayaan 1 Mission
News in the same category


A Parade Moment That Became Global Joy

Nick Vujicic: Living Proof That the Human Spirit Knows No Limits

You’re Made of Stardust – Literally! 🌌🚀

Sea Levels Are Rising Faster Than At Any Time In 4,000 Years 🌍

Your Dog Might Actually Love You More Than Food
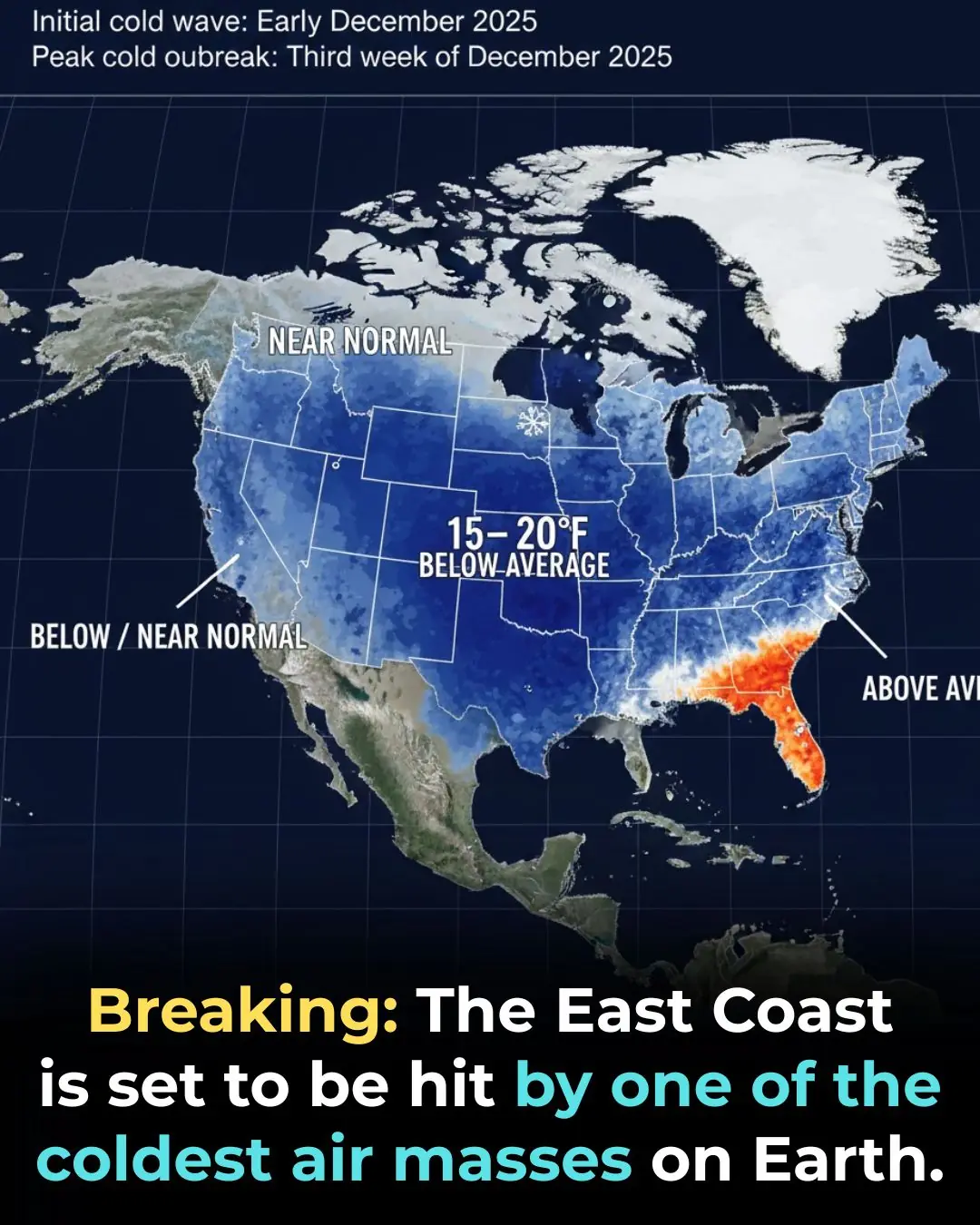
Deep Freeze Set to Slam the Eastern U.S. This December

Nike Co-Founder Phil Knight Makes Historic $2 Billion Donation to Cancer Research

Dutch Engineers Tackle the Pacific’s Plastic Crisis with 600-Meter Ocean Vacuum
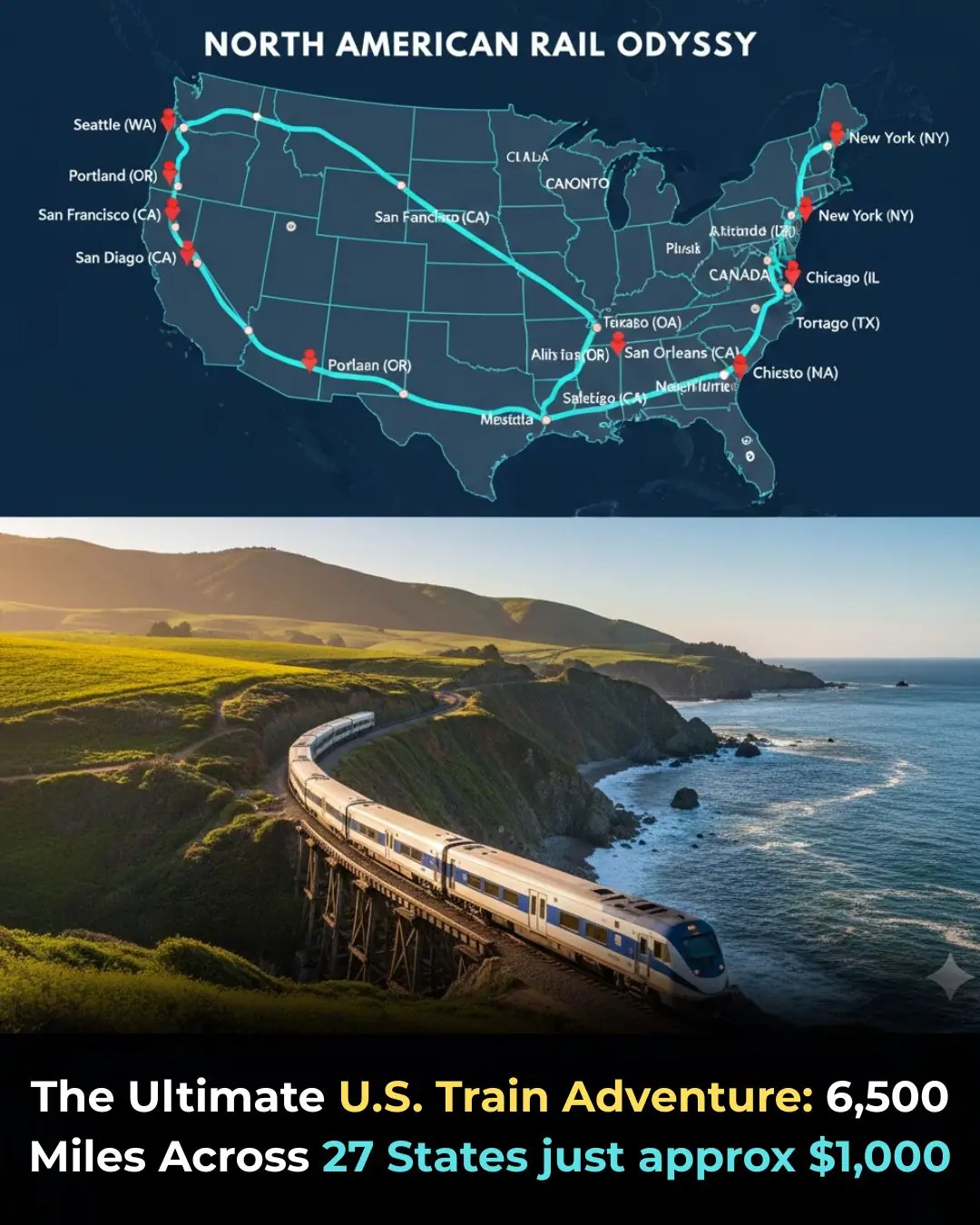
How to Take a Loop of the Entire U.S. by Train

The Quiet Rise of Everyday Health-Tracking Technology
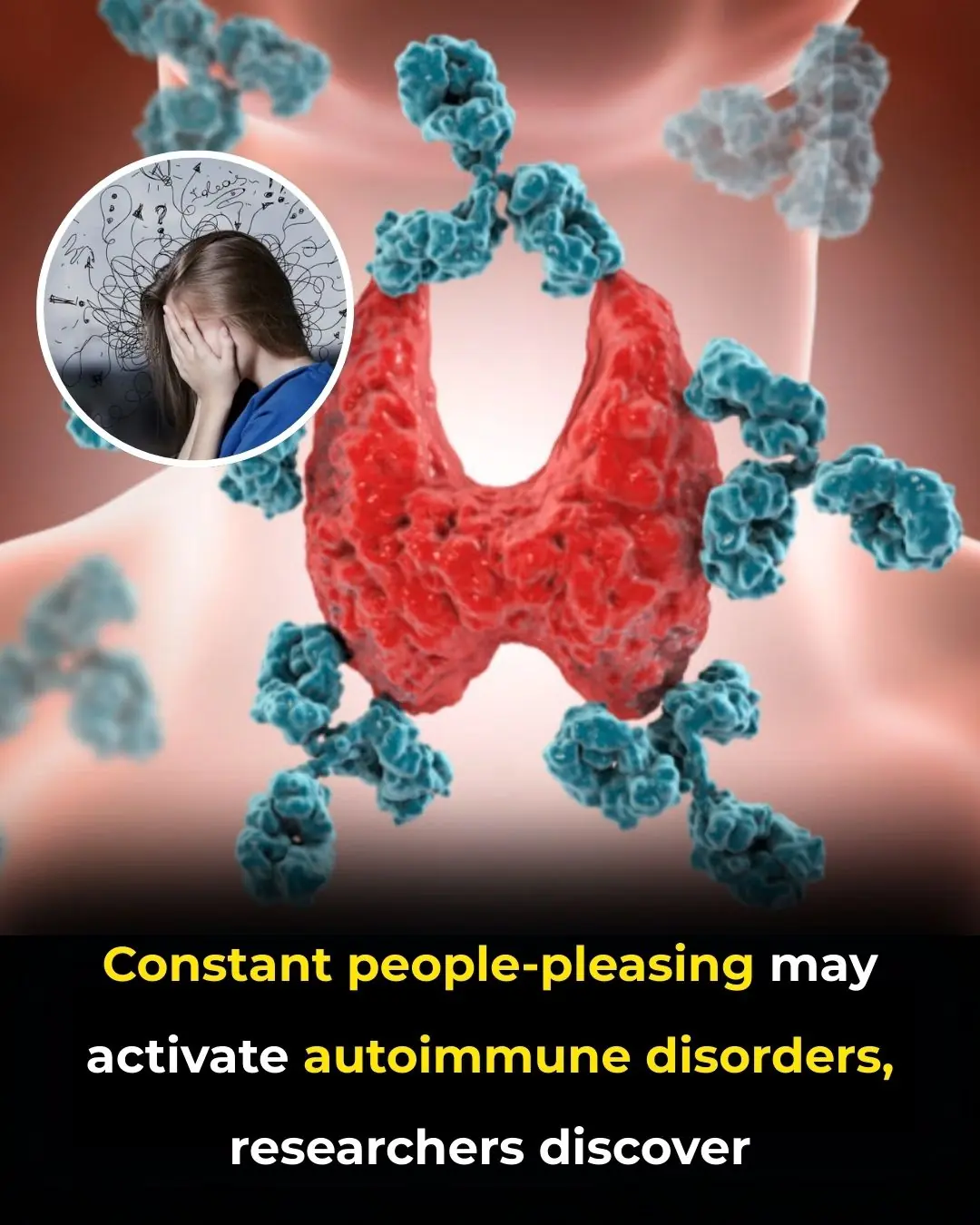
The Hidden Toll of People-Pleasing: How Emotional Suppression Can Trigger Autoimmune Disorders

The Pudu: The World’s Tiniest Deer and Its Role in South America's Forest Ecosystems

Deep Water Cycle: Scientists Discover Hidden Ocean Beneath Earth's Surface

Mexico City’s Sweeping Bullfighting Ban Marks Major Shift in Cultural and Animal-Welfare Policy

Los Angeles County Erases $180 Million in Medical Debt for 39,000 Residents

The 2025 Atlantic Hurricane Season Intensifies: A Heightened Risk for Major Storms
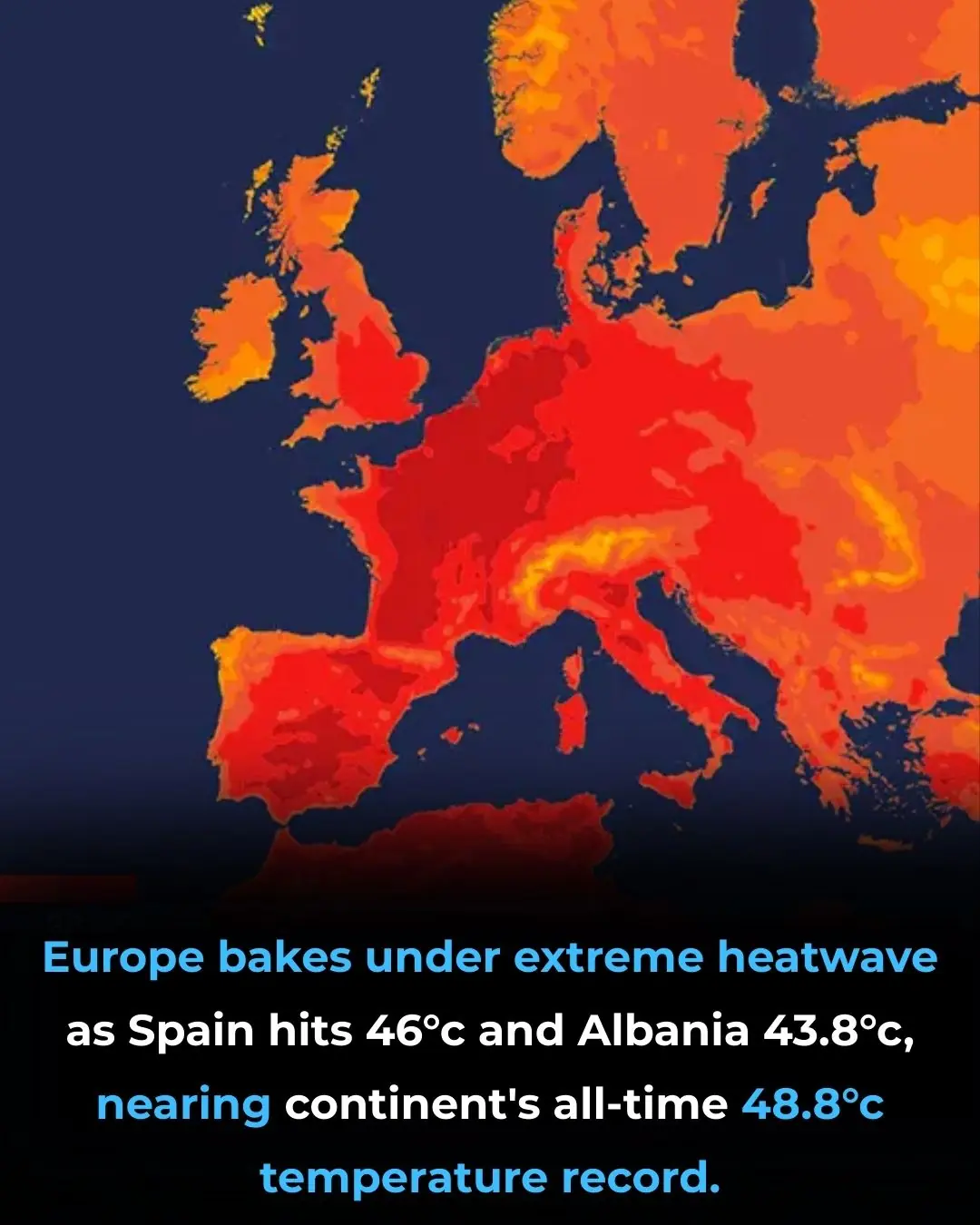
Europe Faces Unprecedented Heatwave: Rising Temperatures Strain People, Infrastructure, and Agriculture

Revolutionary Light-Based Cancer Treatment Offers New Hope with High Success Rate
News Post

Top 8 Foods to Clean and Restore Your Liver Naturally

The Ancient Secret Seed That Revolutionized Wellness: Unlocking the Power of Hibiscus and Cloves

Garlic Remedy for Removing Moles and Skin Tags Naturally: What Works and What to Know

Fibromyalgia: The Hidden Energy Crisis Behind Your Pain, Fatigue, and Sleepless Nights

The Hidden Oil That Sparks Her Desire and Rekindles Your Marriage

Unlock the Ancient Secret of Peach Tree Resin: 15 Life-Changing Benefits You’ll Wish You Knew Sooner

Aloe Vera & Cinnamon: The Traditional Duo That Naturally Supports Your Health, Vitality, and Vision

Why Germany Makes New Dog Owners Take a Test

The Gut-Heart Connection: How Your Gut Microbiome Affects Your Heart Health

One Glass to Flush Your Colon Clean in Just 10 Minutes!

The 11 surprising baking soda uses that actually have science behind them

Common pain meds trick doctors into heart failure misdiagnosis

12 Health Hacks Doctors Rarely Share: Secrets for Optimal Health and Well-being

Top 6 Nutrients To Reduce Knee Osteoarthritis Pain

Could your toes be warning you about your lifespan? Foot expert explains

Could your morning orange juice be supporting your heart more than you think?

Top 10 Signs of Kidney Problems You ABSOLUTELY Must Be Aware Of…

A Parade Moment That Became Global Joy

Nick Vujicic: Living Proof That the Human Spirit Knows No Limits
