
Vitamin K Precursor Destroys Cancer Cells In New Scientific Discovery
In the ever-evolving battle against cancer, scientists tirelessly search for new and better ways to combat this relentless disease. While the focus has long been on antioxidants for their protective qualities, a surprising twist in cancer research is redefining how we might approach treatment in the future. Recent discoveries have highlighted the potential of a seemingly unlikely hero: a vitamin K precursor known not for shielding cells from damage, but for its ability to target and destroy cancer cells. This new research opens a compelling chapter in the quest for effective cancer therapies, suggesting that the key to outsmarting cancer may lie in the very processes that drive its growth.
Understanding Vitamin K Precursors
Vitamin K, a fat-soluble vitamin best known for its critical role in blood clotting, is also essential for bone health and other biological functions. Typically, it’s understood through its forms—phylloquinone (vitamin K1), predominantly found in green leafy vegetables, and menaquinones (vitamin K2), which are synthesized by bacteria in the human gut. However, another lesser-known type, menadione (vitamin K3), serves as a synthetic precursor to vitamin K, primarily used in animal feed and as a nutritional supplement due to its ability to convert into active vitamin K in the bodys out because it also exhibits unique properties not commonly associated with its vitamin counterparts. Unlike the classic roles of vitamins K1 and K2, menadione functions through its pro-oxidant capacity, a characteristic that has recently captured the attention of cancer researchers. Pro-oxidants, contrary to antioxidants, promote oxidation processes, leading to the generation of reactive oxygen species (ROS). These ROS are typically seen as harmful, but in controlled scenarios, they can be harnessed to target and kill cancer cells without damaging healthy tissue.
The transformative potential of menadione in cancer treatment was highlighted in a recent study by Lloyd Trotman at Cold Spring Harbor Laboratory. Trotman and his team discovered that menadione induces oxidative stress specifically in cancer cells, leading to their death. This occurs through a novel mechanism where menadione disrupts the balance of cellular processes that manage cell survival and death. “We are seeing that menadione’s pro-oxidant action can selectively trigger cancer cell death, marking a promising shift in how we might approach cancer therapy,” Trotman remarked in his findings published in Science .
This breakthroerstanding menadione’s dual functionality—as both a vitamin precursor and a pro-oxidant—opens new avenues for therapeutic research. By exploring the properties of vitamin K precursors beyond their conventional roles, researchers are beginning to unravel how these compounds can be repurposed to develop more effective and targeted cancer treatments.
The Shift from Antioxidants to Pro-oxidants in Cancer Research

The shift from focusing primarily on antioxidants to exploring the potential of pro-oxidants in cancer therapy marks a significant pivot in cancer research. Antioxidants, long revered for their ability to neutralize reactive oxygen species (ROS) and reduce oxidative stress, have been a cornerstone in efforts to prevent cellular damage and carcinogenesis. However, the efficacy of antioxidants in cancer treatment has been inconsistent across studies. For instance, while some antioxidants have shown potential in reducing the risk of certain cancers, others have paradoxically been found to accelerate tumor growth and invasiveness under specific conditions.
Pro-oxidants represent a compelling alternative, working on the premise that inducing oxidative stress might selectively kill cancer cells without harming normal cells. This approach stems from the understanding that cancer cells, due to their altered metabolic states, may be more vulnerable to damage from oxidative stress compared to normal cells. Pro-oxidants like menadione, a synthetic form of vitamin K3, have been shown to disrupt cancer cell survival by manipulating cellular redox states and overwhelming the cancer cells’ antioxidant defenses.
Recent research has further highlighted the dual roles of substances like green tea polyphenols, which can act as both antioxidants and pro-oxidants depending on the conditions. This duality suggests that the redox environment within cancer cells might be more complex than previously thought, and that both enhancing and inhibiting oxidation can be therapeutic, depending on the context.
The Discovery of MSB’s Cancer-Fighting Properties
MSB, a vitamin K precursor, has been shown to target a specific lipid kinase in the endosomal pathway known as VPS34. This kinase plays a critical role in cellular trafficking and survival by producing a signaling lipid called phosphatidylinositol 3-phosphate (PI(3)P). MSB disrupts this process by oxidizing specific cysteine residues necessary for VPS34 function, leading to a depletion of PI(3)P levels on the endosomal membrane. As a result, cancer cells fail to sort cellular material properly, leading to cellular burst and death—a process termed “triaptosis” by researchers.
This breakthrough was partly inspired by the unexpected outcomes of the Selenium and Vitamin E Cancer Prevention Trial (SELECT), which showed that antioxidants like vitamin E might increase the risk of prostate cancer rather than reducing it. These findings prompted Trotman to explore the potential of pro-oxidants as a therapeutic strategy, which led to the discovery of the anticancer properties of MSB.
The effectiveness of MSB was demonstrated in mouse models where it significantly slowed prostate cancer progression. This has not only highlighted the compound’s potential for treating prostate cancer but also suggested its efficacy against X-linked myotubular myopathy, a rare and severe muscle disease.
Implications of MSB in Prostate Cancer Treatment
The implications of Menadione Sodium Bisulfate (MSB) in prostate cancer treatment are increasingly promising, showcasing a significant shift towards utilizing pro-oxidant mechanisms to combat cancer. Research led by Professor Lloyd Trotman at Cold Spring Harbor Laboratory has uncovered that MSB, a synthetic form of vitamin K3, effectively halts the progression of prostate cancer in mice by inducing oxidative stress specifically in cancer cells. This is achieved through the depletion of a crucial lipid, phosphatidylinositol 3-phosphate (PI(3)P), which is essential for the cells’ survival processes.
MSB operates by disrupting the cancer cells’ ability to sort and recycle cellular material, ultimately leading to cellular overload and rupture. This mechanism mimics a malfunctioning transport hub, where materials continue to arrive but cannot be properly directed, causing congestion and collapse. This targeted action against cancer cells offers a potential pathway for therapeutic applications that could minimize harm to normal cells, addressing one of the significant challenges in cancer treatment today.
These findings have not only set the stage for pilot studies in human patients but also highlight the broader potential of pro-oxidants in oncology. The research suggests that MSB could be integrated into existing treatment protocols to enhance their efficacy, especially in cases where cancer has shown resistance to conventional therapies. Furthermore, the research on MSB also opens up potential treatment avenues for other conditions, such as X-linked myotubular myopathy, demonstrating the compound’s versatility and the depth of its impact on cellular biology.
Broader Applications and Future Research
The broader implications of Menadione Sodium Bisulfate (MSB) in medical treatment, particularly in prostate cancer, are paving the way for its potential utilization in other diseases that could benefit from pro-oxidant therapy. The research on MSB has highlighted its ability not only to manage prostate cancer but also to explore its efficacy in other complex diseases through the mechanism of inducing oxidative stress selectively in diseased cells without affecting healthy cells.
Future research is aimed at expanding the application of MSB and similar pro-oxidant therapies to include a range of diseases where abnormal cell growth is prevalent, such as in various forms of cancer and potentially in genetic disorders where cellular regulation is disrupted. The exploration into these areas could revolutionize how certain diseases are treated, offering more targeted therapies with potentially fewer side effects compared to traditional treatments.
Researchers are particularly interested in understanding how MSB could complement existing treatments and how its unique mechanism of action could be integrated into broader therapeutic protocols. The potential for MSB to tackle other conditions tied to cellular growth and survival mechanisms opens up new avenues for research and clinical trials that could significantly impact how these diseases are managed in the future.
Further studies and clinical trials will be crucial to determine the safety, efficacy, and broader therapeutic potential of MSB across different medical applications. This ongoing research will help clarify the roles these innovative therapies could play in modern medicine, potentially leading to more effective and personalized treatment options for patients.
Revolutionizing Cancer Treatment with a Pro-Oxidant Approach
The exploration of menadione sodium bisulfate (MSB) in the treatment of prostate cancer marks a pivotal shift in the ongoing battle against this challenging disease. By turning the traditional antioxidant approach on its head, researchers have unveiled the potent cancer-fighting properties of pro-oxidants, highlighting MSB’s ability to selectively induce cancer cell death through oxidative stress. The promising results from preclinical studies not only suggest potential for treating prostate cancer but also open doors to exploring MSB’s effectiveness against other cancers and possibly genetic diseases linked to cellular processing dysfunctions.
As we look towards the future, the potential for MSB to integrate into or enhance existing cancer therapies offers hope for more effective treatments with fewer side effects. The ongoing research into the mechanisms of MSB, alongside clinical trials to confirm its efficacy and safety in humans, is crucial. If successful, MSB could become part of a new generation of cancer treatments, fundamentally changing the way we approach this disease and offering new hope to those affected.
News in the same category

4 Early Symptoms Of Ovarian Cancer That Every Woman Needs To Know

Treating Snake Bites with Papaya Plant

Beware Of Diabetes If You Frequently Experience These 5 Strange Symptoms
10 Signs You’re Living With Clogged Arteries

Doctor ‘Cancer-Free’ One Year After Using Treatment Based On His Own Research

7 Warning Signs of Gynecological Cancer That Women Should Not Ignore

🔥 95-Year-Old Chinese Doctor Drinks THIS Every Day! Liver & Intestines Stay Young! 🍅🥕🍋

10 Cancer-Linked Foods You Should Never Put in Your Mouth Again

Scientists claim to have discovered evidence of 'soul' leaving the body when we die
10 Alarming Signals Your Body Might Be Warning You About
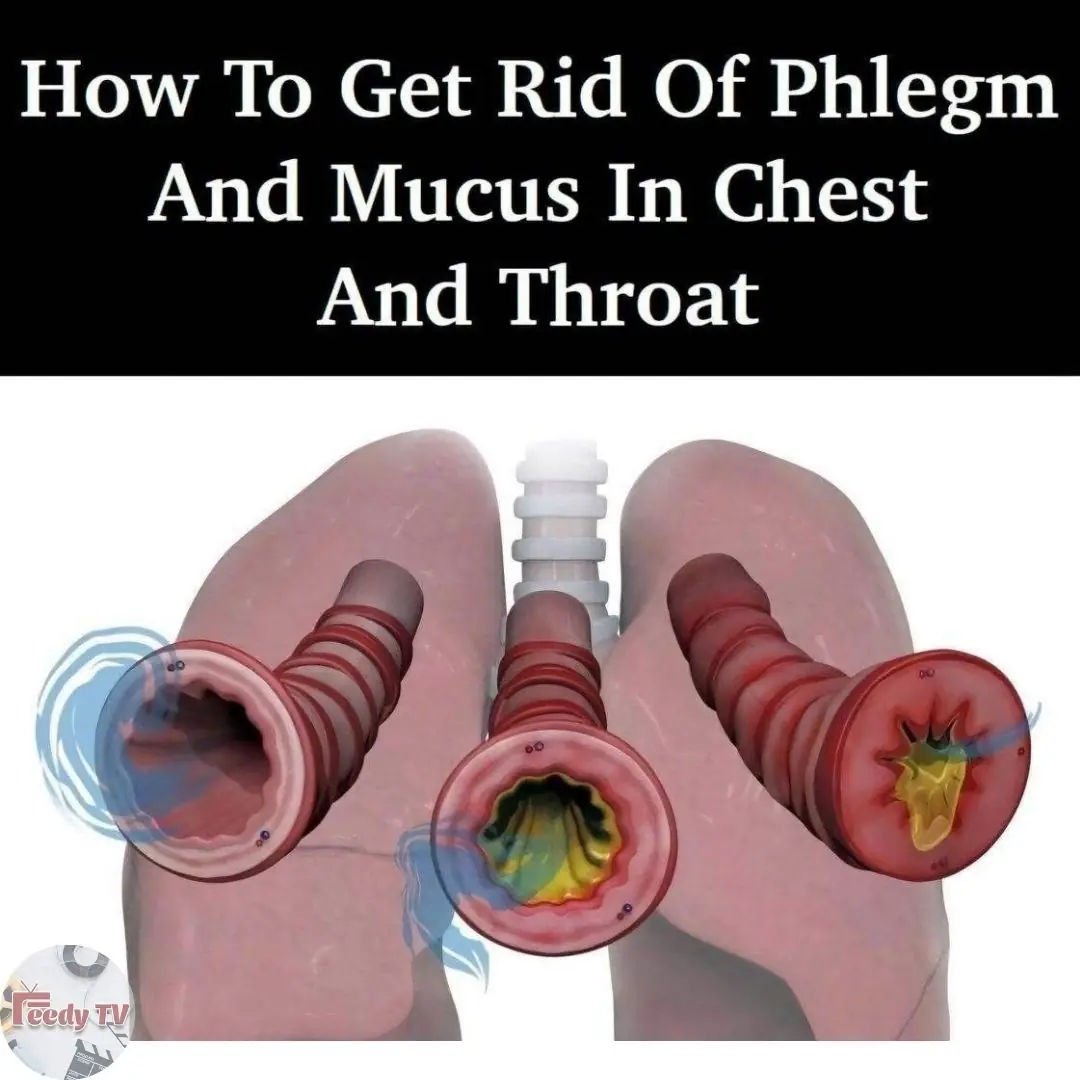
8 Ways To Get Rid Of Phlegm And Mucus In Chest And Throat 👇

The Miracle Herb for Vision: A Natural Solution for Cataracts and Eye Health

High School Student Wins Science Fair – Proves Native American Recipe Kills Cancer Cells IN VITRO

Terrifying simulation shows what happens to your brain when you smoke a cigarette
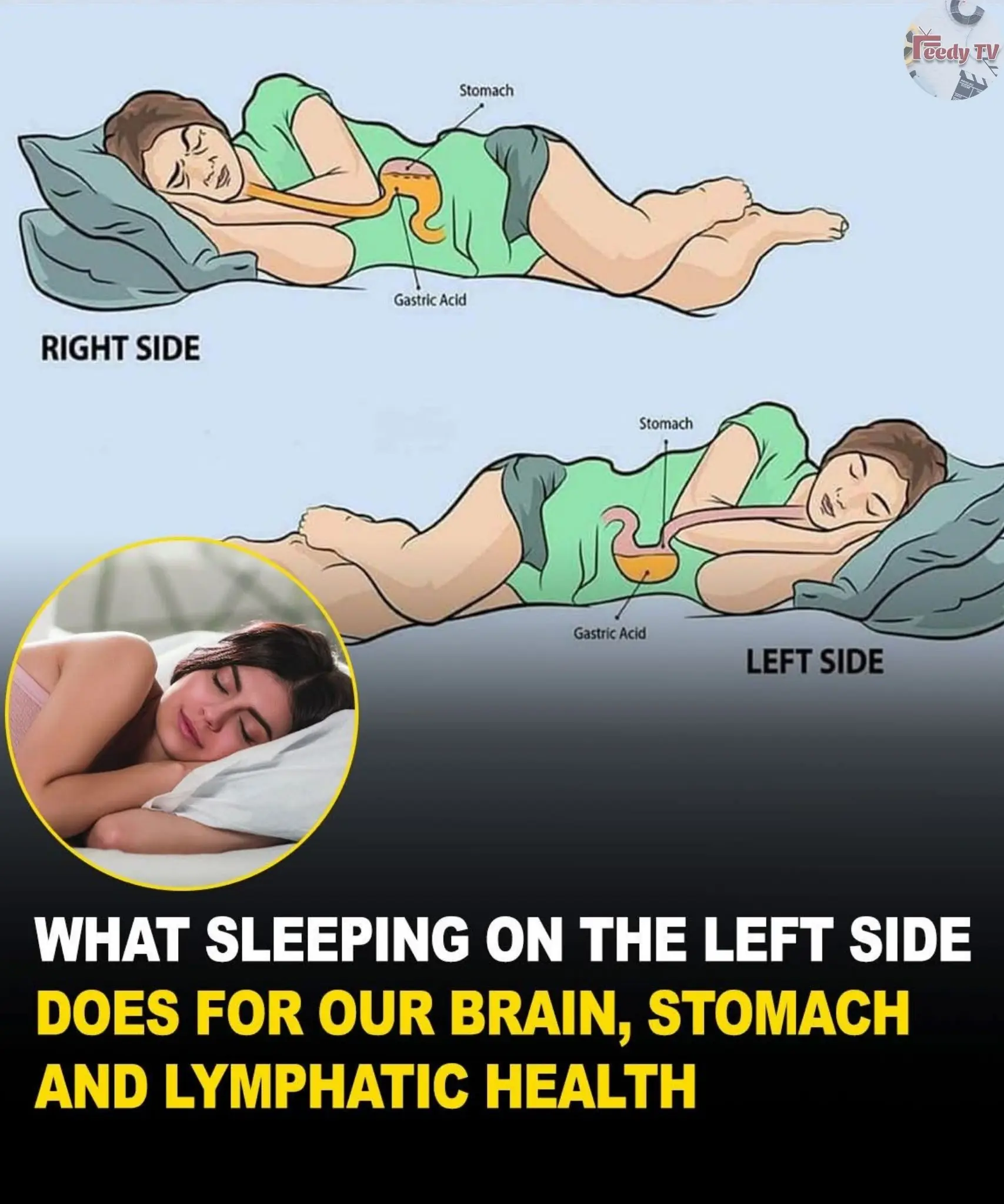
This is what sleeping on the left side does for our brain, stomach & glymphatic health

TOP 18 FAT BURNING Foods Women Should Eat EVERY DAY
Homemade Collagen for Pain, Inflammation, and Joint Health
10 Warning Signs Your Kidneys May Be in Danger
News Post

Lactuca Serriola: The Hidden Power of Prickly Lettuce Growing Everywhere

Carrot Erases All Wrinkles on Your Face! 100-Year-Old Recipe! Top Recipes with Carrots

4 Early Symptoms Of Ovarian Cancer That Every Woman Needs To Know

Treating Snake Bites with Papaya Plant

Beware Of Diabetes If You Frequently Experience These 5 Strange Symptoms

10 Signs You’re Living With Clogged Arteries

Don’t Buy Garlic Anymore! Here’s How to Grow It in a Pot Endlessly

Doctor ‘Cancer-Free’ One Year After Using Treatment Based On His Own Research

7 Warning Signs of Gynecological Cancer That Women Should Not Ignore

Man Constantly Mocks Unemployed Wife for Doing Nothing, Finds a Note after Ambulance Takes Her Away — Story of the Day

Lamb’s Quarters/Wild Spinach:Unveiling the Health and Culinary Wonders

How to Remove Blackheads Naturally

Natural Remedies for Varicose Veins

Hair Growth Juice – Amla Juice & Curry Leaves for Hair

8 Powerful Natural Insecticides to Effectively Combat Your Stubborn Garden Pests

🔥 95-Year-Old Chinese Doctor Drinks THIS Every Day! Liver & Intestines Stay Young! 🍅🥕🍋

10 Cancer-Linked Foods You Should Never Put in Your Mouth Again

The 7 best fertilizers for your tomatoes – they will grow like never before
Secret that Dentists don’t want you to know: Remove Tartar and Teeth Whitening in just 2 minutes
