Revolutionary mRNA Therapy Shows Promise in Preventing Pancreatic Cancer Recurrence
In a landmark breakthrough for pancreatic cancer therapy, researchers have unveiled a novel mRNA‑based treatment that specifically targets tumor‑driving mutations, showing remarkable reductions in recurrence risk in early clinical trials. Pancreatic cancer is widely regarded as one of the most aggressive and treatment‑resistant malignancies, with alarmingly high relapse rates even after surgical resection and chemotherapy.
This pioneering therapy harnesses the power of mRNA technology to instruct the patient’s immune system to identify and attack cancer cells harboring key genetic alterations. By tailoring the mRNA to each tumor’s unique mutation profile, the treatment trains immune cells—especially T cells—to recognize neoantigens, the abnormal proteins produced by mutated cancer genes, and mount a sustained targeted defense. The early-phase results are promising: patients who received this personalized mRNA therapy exhibited significantly lower rates of tumor regrowth, suggesting that the immune system was effectively educated to hunt down and eliminate residual cancer cells.
Experts are optimistic that this development could usher in a new era of precision oncology. Rather than relying on one-size-fits-all chemotherapy, these therapies are customized to the individual’s tumor genetics. This personalisation could substantially reduce side-effects—since the immune system is being directed very specifically—while enhancing the effectiveness of treatment. For a disease like pancreatic ductal adenocarcinoma (PDAC), where survival rates have stagnated for decades, such tailored immunotherapy could be transformative.
The basis for such optimism is supported by recent three-year follow-up data from BioNTech’s Phase 1 trial of their mRNA individualized neoantigen-specific immunotherapy (iNeST), known as autogene cevumeran (BNT122). In this study involving patients whose tumors had been surgically removed, 8 out of 16 participants maintained a robust T‑cell immune response even after three years, and those immune responders enjoyed longer median recurrence‑free survival. GlobeNewswire+1 Moreover, earlier data from a Phase I trial demonstrated that custom mRNA vaccines could be manufactured from a patient’s own tumor tissue, train the immune system, and be safely administered alongside immunotherapy (anti–PD-L1) and chemotherapy. PubMed
Further supporting this personalized approach, a small clinical study led by Memorial Sloan Kettering Cancer Center (MSKCC), funded by the National Institutes of Health (NIH), showed that custom mRNA vaccines targeting up to 20 patient-specific neoantigens provoked a strong immune response in half of the treated participants. National Institutes of Health (NIH)+2Memorial Sloan Kettering Cancer Center+2 Currently, new trials are underway: for example, the University of Birmingham has initiated Europe’s first neoantigen mRNA vaccine trial for post‑surgery pancreatic cancer patients, delivering mRNA tailored to each patient's tumor profile in combination with standard chemotherapy. University of Birmingham In addition, there is an ongoing clinical trial assessing a personalized tumor neoantigen mRNA vaccine (iNeo‑Vac‑R01) plus a PD‑1 antibody and chemotherapy as adjuvant therapy after surgery. npcf.us
The implications of these findings are profound. If further trials confirm that personalized mRNA vaccines can substantially delay or prevent cancer relapse, this could become a vital weapon in the clinical arsenal against pancreatic cancer—transforming a historically lethal disease into one that can be more effectively controlled or even cured. Moreover, the success of this approach highlights the broader promise of mRNA technology well beyond vaccines against infectious diseases, extending into highly personalized oncology.
However, there remain challenges. Manufacturing custom mRNA for each patient is complex, costly, and time-sensitive; there is also the risk that tumors may evolve or escape immune detection. As commentators have noted, large-scale, randomized trials are still needed to establish long-term safety, consistency, and clinical benefit. PubMed Nevertheless, the early evidence offers hope—not just for pancreatic cancer patients, but for the future of individualized cancer immunotherapy as a whole.
References
-
BioNTech three-year follow-up on iNeST Phase 1 trial in PDAC. GlobeNewswire+1
-
Phase I trial of autogene cevumeran in pancreatic cancer. PubMed
-
Personalized mRNA vaccine development at MSKCC. Memorial Sloan Kettering Cancer Center
-
NIH‑funded research on mRNA neoantigen vaccine in PDAC. National Institutes of Health (NIH)
-
University of Birmingham’s first neoantigen mRNA vaccine trial. University of Birmingham
-
Clinical trial of iNeo‑Vac‑R01 personalized mRNA + PD‑1 + chemotherapy. npcf.us
-
Commentary on challenges of personalized mRNA cancer vaccines. PubMed
News in the same category


Reviving the Brain’s Waste-Clearing Pathways May Reverse Alzheimer’s Damage

New CRISPR Therapy Shows Promise in Removing HIV and Preventing Viral Rebound
Japanese Scientists Develop Drug That Could Regrow Human Teeth

White House Gives Major Update On Donald Trump's

What’s with the white paint on the tree trunks?

If You See A Bent Tree In The Forest, Start Looking Around Immediately

Scientists Discovered A Sinkhole 630 Feet Underground In China Known As “Heavenly Pits”

Ever Wake Up But Can’t Move

Are Eggs with Bl00d Spots Safe to Eat? The Truth Behind Those Tiny Red Specks

You’ll Think Twice About Soft Serve

A 42-Year-Old Man Died of a Stroke Despite Not Smoking or Drinking — Doctors Shocked to Find the Real Daily “Killer” in His Diet

How to Remove Water Stains from Wood with Mayonnaise

Close or open the bathroom door for ventilation? It turns out many people still do it wrong

What Does It Mean To Wear a Ring On The Right Hand

Who Will Not Be Eligible As Trump Promises To Give $2,000 To Almost Everyone In America

I Thought I Found Insect Eggs Under My Bed

The Lip Color You Pick Reveals What Kind of Woman You Are
News Post

16-Pound Giant Baby Made Headlines In 1983, But Wait Till You See Him Today

Research reveals the #1 vitamin for eye protection

World’s Most Secret Underground Villa Built By A Woman Living Off The Grid

Don’t Rinse Pork Immediately After Buying It — Use This Method to Keep It Fresh and Delicious for a Whole Month

Don’t Clean Your Rice Cooker with Plain Water: Use This Solution — Sparkling Clean in Just 5 Minutes

My nana taught me this hack to deodorize trash cans in 2 mins with 0 work. Here’s how it works

Neat Hack

Boiling Shrimp in Plain Water Makes Them Fishy and Mushy — Add This for Bright Red, Firm, Flavorful Shrimp

I Had No Idea What That Little Fabric Square Was For — Until Now

Girl Abandoned By Parents For Her Looks Wants To Prove The World Wrong – Now She Models For Vogue

12 Weird but Genius Ways to Unclog Things Naturally

Don’t Eat Tofu Right After Buying It — Freeze It First! The Results Are Surprisingly Amazing

Put Borax on Wax Paper and Slide It Under Your Fridge — Here’s Why

10 Surprising Ways to Use Vinegar Around the House

Works Like a Charm

My nana taught me a brilliant 2-minute hack that makes dusty blinds sparkle with zero effort — here’s how it works

Woman Turns Boeing Plane Into Fully Functional Home
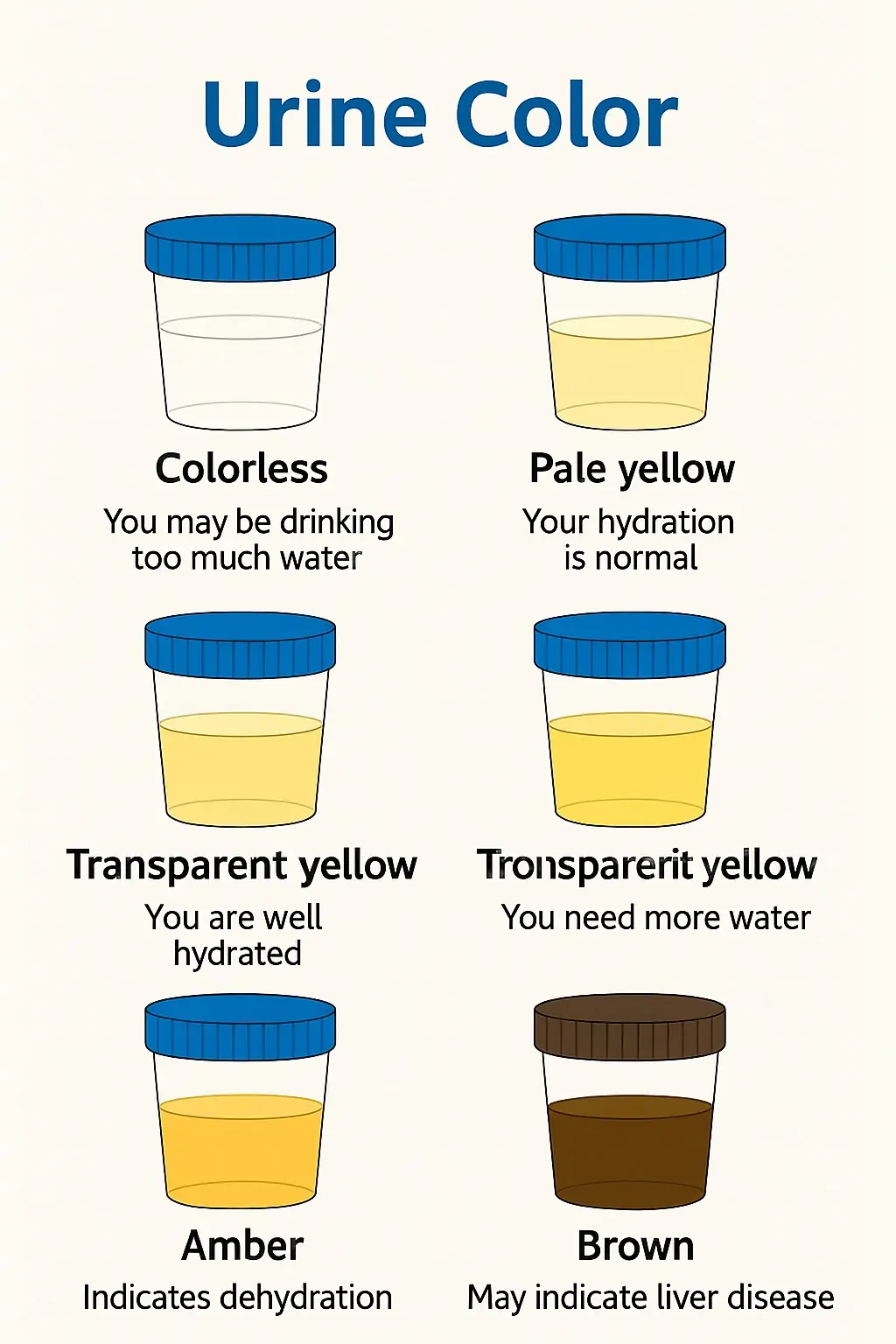
What Your Urine Color May Be Telling You (Gently & Naturally)