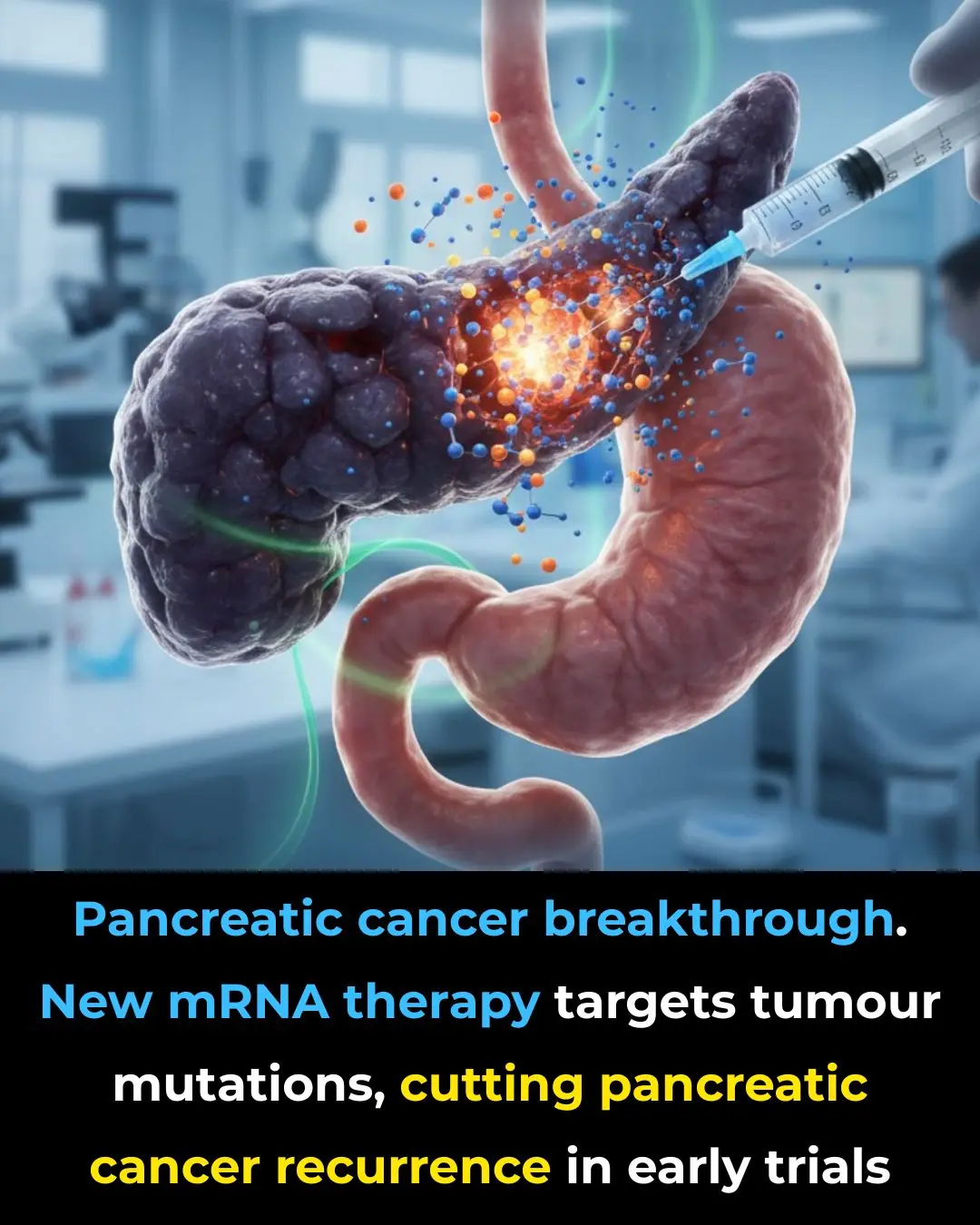
MIT Scientists Develop Injectable Gel That Can Fully Repair Nerves and Restore Sensation
Scientists at MIT have reportedly developed a groundbreaking injectable gel capable of repairing damaged nerves and fully restoring sensation. In laboratory experiments, the gel acted as a scaffold, promoting nerve regrowth: injured neurons were able to reconnect and gradually reestablish their natural function. Over time, this innovation could fundamentally change how serious nerve injuries are treated.
Traditionally, nerve damage often leads to severe and long-lasting consequences — permanent loss of sensation, chronic pain, or even paralysis — because regrowing nerves lack both a guiding structure and the correct target pathways. This new injectable gel, however, does more than simply support nerve regrowth. It appears to offer a twofold benefit: creating a nourishing environment for neurons to regrow, and importantly, guiding regenerating nerve fibers toward their proper destinations. In doing so, it dramatically improves the chances of functional recovery.
If successfully translated into clinical use, this technology could have major implications for patients suffering from spinal cord injuries, traumatic nerve injuries (such as from accidents), and even some neurological conditions. Researchers are optimistic that, in the future, this gel-based therapy may enable patients to regain not only sensory awareness — the ability to feel touch, pressure, or temperature — but also motor control, restoring full sensory and motor functionality.
This achievement underscores the remarkable power of bioengineering. By developing materials that communicate with the body at a cellular level, science is pushing the boundaries of tissue regeneration and repair. What was once considered permanent nerve damage may soon become a treatable condition — a restoration of function once thought impossible.
Context & Supporting Research
While the description above captures the promise of the MIT innovation, it aligns with and is reinforced by a growing body of research in injectable biomaterials and hydrogels for neural repair:
-
A recent study demonstrated an injectable nerve-specific hydrogel that, when injected into a rat model of sciatic nerve crush, significantly improved functional recovery without adverse effects, showing both safety and efficacy. PubMed
-
Other researchers have developed injectable hydrogels containing growth‑factor mimetic peptides (such as VEGF-mimetic peptides) carried by nanoliposomes. In peripheral nerve injury models, these gels have promoted revascularization, polarized macrophages to a pro-regenerative phenotype, and enhanced axon regrowth and remyelination. PubMed
-
Even more specialized conductive hydrogels are under exploration: for example, scientists have synthesized a stretchable, electrically conductive hydrogel (based on polyaniline and polyacrylamide) that restored bioelectrical signaling and improved functional recovery in nerve-injured animal models. ScienceDaily+1
-
In another branch of research, an injectable conductive polymer hydrogel made from PEDOT (poly(3,4-ethylenedioxythiophene)) was shown to support regeneration of the optic nerve, leading to restored visual electrophysiological responses and better survival of retinal ganglion cells in animal models. PubMed
-
More broadly, bioengineers at MIT (and collaborators) have also worked on computational frameworks for designing injectable granular hydrogels, which help predict the physical behavior (e.g., injectability) of these materials, making them more viable for clinical translation. news.mit.edu
Potential and Future Challenges
Despite the promise, there are still significant hurdles to overcome before such an injectable nerve-regeneration gel can become a routine treatment:
-
Translational Barriers: Results in animals do not always translate directly to humans. Large-animal studies and clinical trials will be required to confirm safety, efficacy, and long-term outcomes.
-
Immune Response: Any biomaterial introduced into the body provokes an immune response. Ensuring the gel remains biocompatible and does not trigger inflammation or fibrosis is critical.
-
Precision Targeting: Guiding regenerating axons to the correct targets is a non-trivial challenge, especially in complex injury sites. The engineering of the scaffold must ensure directional guidance without miswiring.
-
Regulatory Approval: For clinical adoption, such materials must go through rigorous regulatory pathways (e.g., FDA approval), which require extensive safety and manufacturing validations.
Why This Matters
If successful in clinical settings, this injectable gel could revolutionize the treatment of nerve injuries. For patients, it means hope for regaining lost function without invasive, complex surgeries. For medicine, it represents a new paradigm: rather than simply managing nerve damage, we could actively heal nerves by leveraging bioengineered materials. Bioengineering is not just patching damage — it's offering a restorative scaffold that enables the body to rebuild itself.
News in the same category


A New Era of Computing: China’s Quantum Machine Surpasses the World’s Fastest Supercomputers

Reviving the Brain’s Waste-Clearing Pathways May Reverse Alzheimer’s Damage

New CRISPR Therapy Shows Promise in Removing HIV and Preventing Viral Rebound
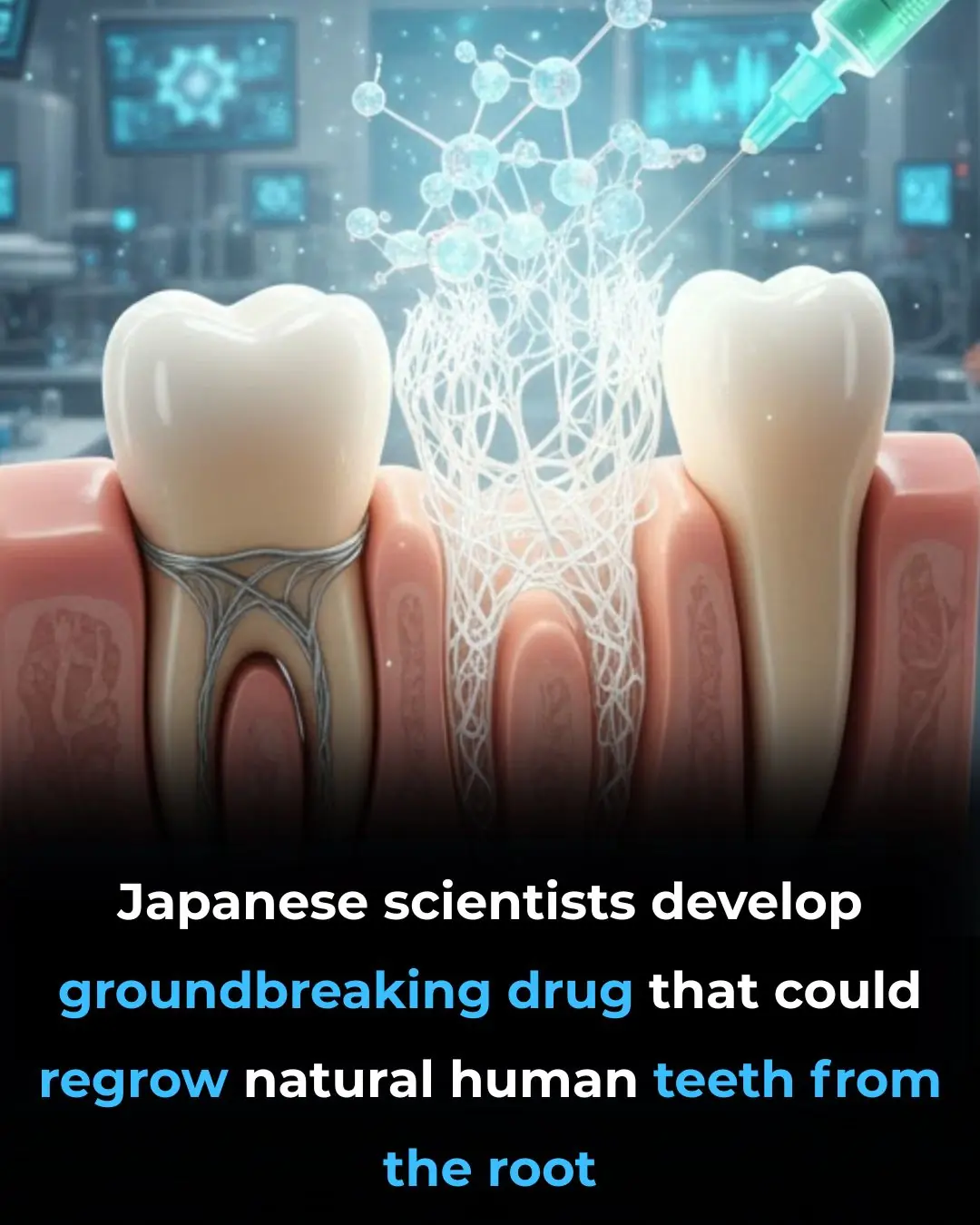
Japanese Scientists Develop Drug That Could Regrow Human Teeth

White House Gives Major Update On Donald Trump's

What’s with the white paint on the tree trunks?

If You See A Bent Tree In The Forest, Start Looking Around Immediately

Scientists Discovered A Sinkhole 630 Feet Underground In China Known As “Heavenly Pits”

Ever Wake Up But Can’t Move

Are Eggs with Bl00d Spots Safe to Eat? The Truth Behind Those Tiny Red Specks

You’ll Think Twice About Soft Serve

A 42-Year-Old Man Died of a Stroke Despite Not Smoking or Drinking — Doctors Shocked to Find the Real Daily “Killer” in His Diet

How to Remove Water Stains from Wood with Mayonnaise

Close or open the bathroom door for ventilation? It turns out many people still do it wrong

What Does It Mean To Wear a Ring On The Right Hand

Who Will Not Be Eligible As Trump Promises To Give $2,000 To Almost Everyone In America

I Thought I Found Insect Eggs Under My Bed

The Lip Color You Pick Reveals What Kind of Woman You Are
News Post

THE CAT THAT BR0KE ALL THE RULES: A POLICE OFFICER'S UNEXPECTED MISSION

Mullein: Exploring the Benefits of Leaves, Flowers, and Roots

Airport X-Ray Scanners Upgrade: Shocking Level of Details

From White Hair to Naturally Darker Hair: Fast Home Remedies & Growth Tips

Teen told he just had ‘growing pains’ dies day after diagnosis

7 Ways To Use Vaseline For Wrinkle Free, Flawless Skin

EVERYTHING JAMES FRANCO SAID ABOUT BEING ‘CAST OUT’ FROM HOLLYWOOD DURING HIATUS

The #1 seed that makes bones & muscles strong—how to use it!

14 Warning Signs of Low Magnesium Levels and What to Do About It (Science Based)

Strictly Come Dancing star eliminated from competition on their birthday
Top 10 Foods to Heal Knee Pain and Boost Cartilage Naturally

Blood Type O Diet: What to Eat and What to Avoid
7 nutrients that actually repair nerves

Coronation Street's Lucy Fallon shows off huge ring as she announces engagement

MAFS UK’s ‘strongest’ couple split after romantic display at reunion

The Versatility and Benefits of Orange Peel Powder

The Hidden Power of the Honey Locust Tree (Gleditsia triacanthos): Health, Healing, and Everyday Uses

This one vitamin could help stop you from waking up to pee every night

The Cold Room Sleep Trick That Can Transform Your Health
