
BREAKTHROUGH: FDA Approves First New Gonorrhea Antibiotics in Over 30 Years—A Game-Changer in the Fight Against Drug Resistance
A Medical Breakthrough After Decades: FDA Approves New Antibiotics to Treat Gonorrhea
In a monumental development that marks a significant victory in the battle against antibiotic-resistant infections, the U.S. Food and Drug Administration (FDA) has approved new antibiotics for the treatment of gonorrhea for the first time in over 30 years. This milestone offers new hope in the ongoing fight against drug-resistant infections, particularly as gonorrhea has evolved and become increasingly difficult to treat.
The Growing Threat of Drug-Resistant Gonorrhea
Gonorrhea, a sexually transmitted infection (STI) caused by the Neisseria gonorrhoeae bacterium, has long been a significant public health challenge. Over the years, this infection has grown more resistant to commonly used antibiotics, leading to fears that existing treatment options might soon become ineffective. According to the Centers for Disease Control and Prevention (CDC), gonorrhea is now one of the most frequently reported STIs in the world, with over 80 million cases annually.
The rise of antibiotic resistance in gonorrhea has been alarming. The bacteria have developed resistance to nearly every class of antibiotics used to treat the infection, including penicillins, tetracyclines, and fluoroquinolones. This escalation has led to concerns among public health experts, who have repeatedly warned that treatment options were becoming dangerously limited, and the spread of the infection was increasingly difficult to control.
New Hope in the Fight Against Resistance
After years of limited options, the approval of new oral antibiotics represents a critical breakthrough in the fight against gonorrhea. These new drugs employ innovative mechanisms designed to outsmart the resistant strains of the bacteria. Unlike previous treatments, which often relied on a single injectable antibiotic, these newly approved oral antibiotics provide a more accessible and effective alternative, particularly for patients in regions where healthcare access is limited.
Experts believe that this development could mark a major shift in gonorrhea treatment. Previously, the only treatment options were an injectable drug used for decades, but the approval of new oral antibiotics offers more flexibility and accessibility, especially for patients who have difficulty accessing injectable medications. The availability of these new drugs could also streamline treatment regimens, making it easier for healthcare providers to offer care on a broader scale..jpg)
Implications for Public Health
The approval of these new antibiotics is not just a win for individuals affected by gonorrhea but also a significant step forward in the global fight against antimicrobial resistance (AMR). Public health experts emphasize that this breakthrough could slow the spread of resistant infections, helping to preserve the effectiveness of antibiotics in general. Given the increasing rates of gonorrhea cases worldwide, particularly in areas with limited access to healthcare, the new treatments could prove invaluable in controlling the spread of the infection.
The CDC has long warned that antimicrobial resistance is one of the greatest public health threats of our time, as it complicates the treatment of various infections. The introduction of these new antibiotics provides a much-needed tool in this global effort, particularly as antibiotic resistance continues to spread across the globe.
Global Impact: Accessibility and Prevention
In addition to its potential to slow resistance, the new antibiotic treatment could also make gonorrhea treatment more accessible on a global scale. Gonorrhea cases have been rising globally, with particularly concerning increases in regions like sub-Saharan Africa, Eastern Europe, and parts of Asia. The ability to treat gonorrhea with oral antibiotics—rather than relying solely on injectable medications—could facilitate treatment in low-resource settings where healthcare infrastructure is often lacking.
This approval represents not just a technical advancement but also a policy victory for public health initiatives. Access to effective treatment is essential to preventing further transmission of gonorrhea and reducing its burden on individuals and healthcare systems. Experts agree that improving global access to these new antibiotics will be a key factor in controlling the spread of gonorrhea and similar diseases in the coming years.
Looking Ahead: The Future of Gonorrhea Treatment
While the approval of these new antibiotics is certainly a step forward, it is not a permanent solution. Experts stress the importance of continued research into novel treatments for gonorrhea and other antibiotic-resistant infections. The World Health Organization (WHO) and CDC both highlight the urgency of developing new antibiotics and treatments to stay ahead of rapidly evolving bacterial strains. Additionally, continued prevention efforts such as widespread education on safe sex practices and regular STI screening will be essential in curbing the spread of gonorrhea and other STIs.
There are also calls for a greater emphasis on global cooperation in the fight against antimicrobial resistance, as drug-resistant infections do not respect national borders. The approval of new treatments is only one piece of the puzzle—ensuring that these drugs reach the people who need them most will be crucial to their success in controlling gonorrhea globally.
Conclusion: A New Era in Gonorrhea Treatment
The FDA’s approval of new antibiotics for gonorrhea marks a new era in the fight against antibiotic resistance. This milestone offers hope not only for better treatment options but also for the possibility of preventing the further spread of drug-resistant gonorrhea. However, this development is just the beginning. Public health experts, healthcare providers, and policymakers will need to work together to ensure that these new treatments are widely accessible and that continued efforts to combat antimicrobial resistance remain a global priority.
Sources and Further Reading
-
Centers for Disease Control and Prevention (CDC) - Gonorrhea Statistics and Resistance Information. CDC website
-
World Health Organization (WHO) - Antimicrobial Resistance: Global Report on Surveillance. WHO website
-
The Lancet Infectious Diseases - The Global Challenge of Gonorrhea and Antimicrobial Resistance. Lancet Infectious Diseases
News in the same category


Vanity Items Became Collectible Art

California to Require Seat-Belt Fit Test for Kids and Teens Before Switching from Booster Seats Starting 2027
Revolutionary mRNA Lung Cancer Vaccine Begins Human Trials—A Breakthrough in Cancer Treatment

Shaquille O'Neal’s Accidental Investment in Google Turned into a Fortune

UC San Francisco Researchers Discover Method to Transform White Fat into Calorie-Burning Beige Fat, Offering New Hope for Obesity Treatment

Japan Launches Groundbreaking Human Trial for Tooth Regeneration—Could We Soon Regrow Our Own Teeth?

Dolphins Join Elite Group of Animals with Self-Recognition, Challenging Our Understanding of Animal Intelligence

12 Signs You Have a Spiritual Gift (Even If You Don’t Realize It Yet)

Children Who Are Hugged Often Have Stronger Immune Systems, Studies Show

Prenatal Music Boosts Brain Development in Newborns—Find Out How It Shapes Early Language Skills
Revolutionary Gene-Editing Treatment Could Permanently Lower Cholesterol by 50%—No More Daily Medications!

Meet the Oldest Ribbon Worm Ever: This 30-Year-Old Marine Creature Is Shaking Up Science

Little Pocket in Women’s Underwear

Fabric Softeners and Indoor Air Quality: Hidden Health Risks You Should Know
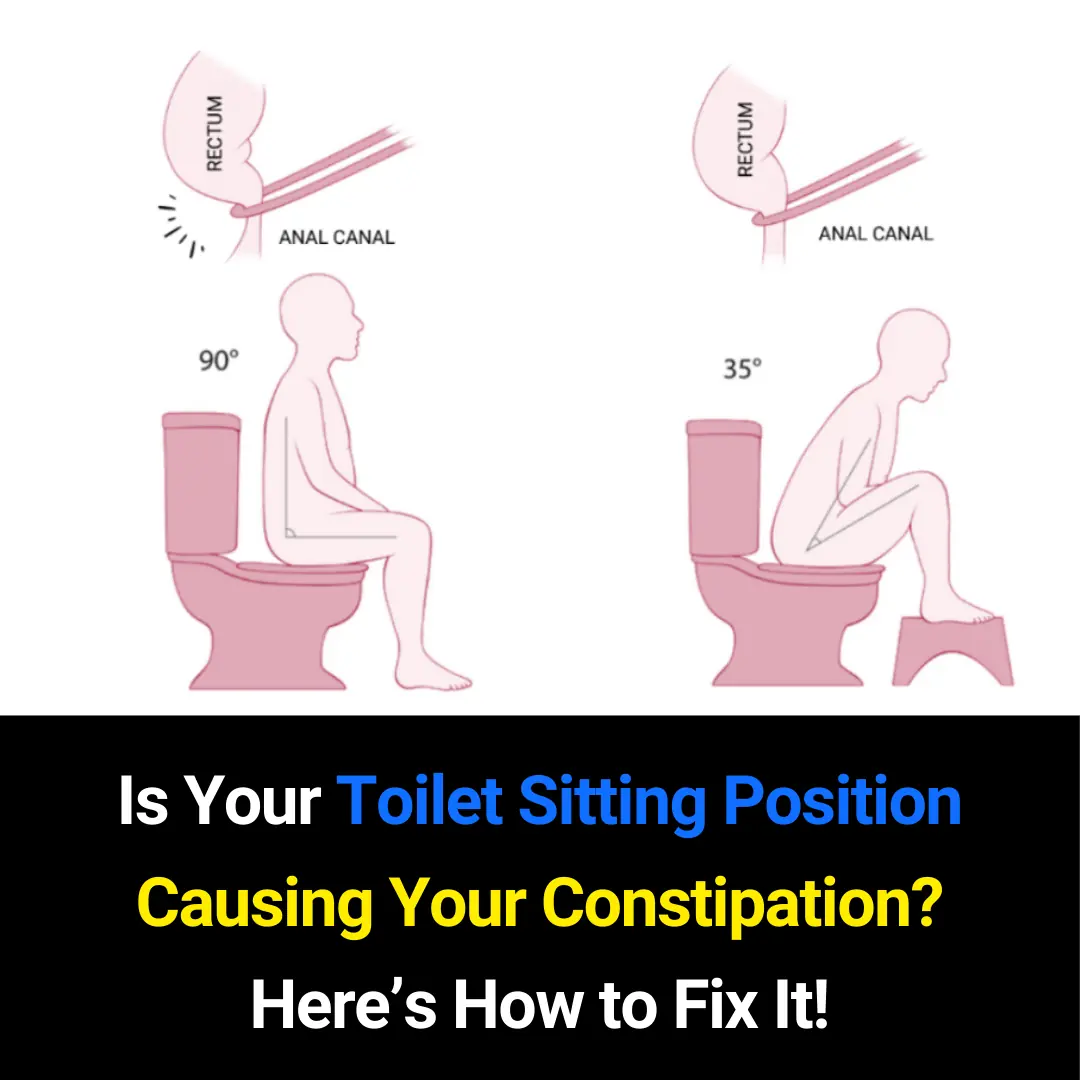
Is Your Toilet Sitting Position Causing Constipation? Here’s How to Fix It Naturally
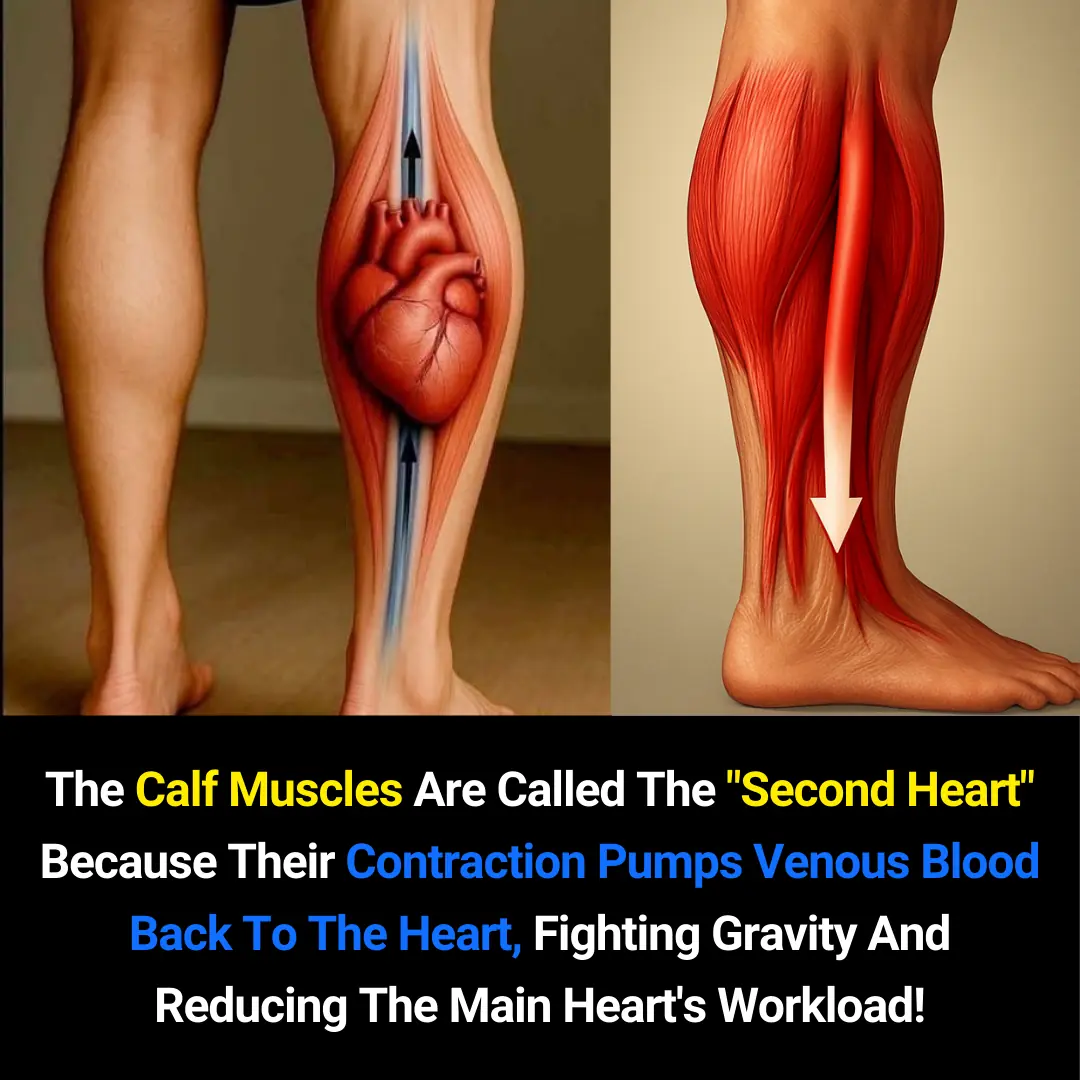
The Calf Muscles: Why They Are Known as the Body’s “Second Heart”

Little Pocket in Women’s Underwear

A Decade of Struggle: The Inspiring Journey from Wrongful Conviction to Legal Triumph
News Post

Surprising Benefits of Sitting Facing Forward on the Toilet

Vanity Items Became Collectible Art

California to Require Seat-Belt Fit Test for Kids and Teens Before Switching from Booster Seats Starting 2027

Revolutionary mRNA Lung Cancer Vaccine Begins Human Trials—A Breakthrough in Cancer Treatment

Shaquille O'Neal’s Accidental Investment in Google Turned into a Fortune

UC San Francisco Researchers Discover Method to Transform White Fat into Calorie-Burning Beige Fat, Offering New Hope for Obesity Treatment

Japan Launches Groundbreaking Human Trial for Tooth Regeneration—Could We Soon Regrow Our Own Teeth?

Dolphins Join Elite Group of Animals with Self-Recognition, Challenging Our Understanding of Animal Intelligence

12 Signs You Have a Spiritual Gift (Even If You Don’t Realize It Yet)

Children Who Are Hugged Often Have Stronger Immune Systems, Studies Show

Fatty Liver Disease: 6 Warning Signs You Should Never Ignore
10 Vitamins and Minerals You Should Never Take — and Why

Prenatal Music Boosts Brain Development in Newborns—Find Out How It Shapes Early Language Skills

Revolutionary Gene-Editing Treatment Could Permanently Lower Cholesterol by 50%—No More Daily Medications!

Meet the Oldest Ribbon Worm Ever: This 30-Year-Old Marine Creature Is Shaking Up Science

DIY Collagen Powder Recipe for Glowing Skin & Thick Hair

Clove benefits for Skin – Clove Oil, Clove Gel & Clove ice cubes

Clove benefits for Skin – Clove Oil, Clove Gel & Clove ice cubes
