
Revolutionary mRNA Lung Cancer Vaccine Begins Human Trials—A Breakthrough in Cancer Treatment
A Game-Changing Medical Milestone: The Development of the First mRNA-Based Lung Cancer Vaccine
In an exciting leap forward for cancer treatment, a revolutionary mRNA-based lung cancer vaccine, known as BNT116, has officially entered clinical trials across seven countries, including the United States, the United Kingdom, and Germany. This groundbreaking development is poised to mark a new era in cancer immunotherapy, offering hope for more effective, targeted treatments.
The Power of mRNA Technology in Cancer Treatment
Developed by BioNTech, the same company behind the highly successful COVID-19 vaccine, BNT116 utilizes the same mRNA technology that has already proven to be a game-changer in global healthcare. The vaccine is designed to train the immune system to recognize and attack lung cancer cells. Unlike traditional cancer treatments, which rely on chemotherapy, radiation, or surgery, BNT116 offers a novel approach by using the body’s natural defense system to fight cancer.
The concept behind using mRNA technology for cancer treatment is rooted in the ability to teach the immune system to detect and destroy cancer cells more effectively. By introducing specific genetic material into the body, the vaccine essentially provides the immune system with a "blueprint" to identify cancer cells as a threat and mount a targeted attack against them.
The Clinical Trials: A Global Effort
The first phase of clinical trials for BNT116 has already begun, with a 67-year-old man from London becoming the first patient to receive the vaccine. This milestone is a significant moment in the development of personalized cancer immunotherapy, as it represents the beginning of a potential new era where cancer treatment is tailored to each individual’s immune system.
The vaccine’s trials are being conducted across seven countries, including major locations in the UK, US, and Germany, and involve a target group of 10,000 patients by the year 2030. This wide-scale approach aims to evaluate the vaccine's safety, efficacy, and potential to induce immune responses in patients with non-small cell lung cancer (NSCLC), the most common type of lung cancer. Lung cancer remains one of the deadliest cancers globally, making this research an urgent and potentially life-saving breakthrough.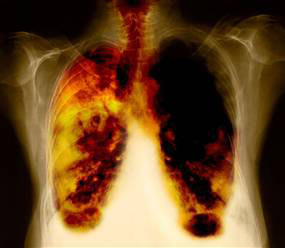
A Step Toward Personalized Cancer Immunotherapy
BNT116’s entry into clinical trials signals a bold step toward the future of personalized cancer treatment. Instead of relying on generalized treatments, personalized cancer therapies like this one aim to create a more targeted, individualized approach to treatment. By tailoring treatments to the specific genetic profile of the cancer and the patient’s immune system, it is hoped that the effectiveness of the treatment will be significantly increased while minimizing harmful side effects.
This approach aligns with the broader field of immunotherapy, which has shown tremendous promise in the treatment of various cancers. The success of therapies like checkpoint inhibitors has already demonstrated that stimulating the body’s immune system can result in significant responses against cancer. With mRNA technology, researchers believe they can further enhance this approach by teaching the immune system to directly target cancer cells in a much more specific and effective manner.
The Potential to Revolutionize Cancer Treatment
If the clinical trials are successful, the BNT116 vaccine could represent a monumental shift in how we approach cancer treatment. Traditional cancer treatments, such as chemotherapy and radiation, work by attacking rapidly dividing cells, but they often come with severe side effects because they also affect healthy cells. In contrast, immunotherapy seeks to harness the body’s natural defenses, leading to more precise targeting of cancer cells.
Moreover, the advent of mRNA vaccines for cancer opens up new possibilities for the treatment of other types of cancer as well. If BNT116 proves successful in treating lung cancer, similar vaccines could potentially be developed for other cancers, such as breast cancer, melanoma, and colon cancer. This would represent a major step forward in the fight against cancer, transforming it from a disease largely defined by aggressive, invasive treatments to one that could be fought with precision medicine.
Looking to the Future: Expanding the Reach of mRNA Cancer Vaccines
The trial is part of BioNTech’s broader commitment to harnessing mRNA technology for a wide range of therapeutic uses beyond COVID-19. As mRNA technology continues to show its potential in vaccine development, researchers are exploring its use in a variety of other medical fields, including infectious diseases, autoimmune conditions, and of course, cancer.
This innovative approach could revolutionize cancer treatment, offering not only new hope for those with lung cancer but also the possibility of extending this technology to a broader range of cancers. If successful, the BNT116 vaccine could serve as a template for future mRNA-based cancer vaccines, potentially transforming the landscape of oncology.
Challenges and the Path Forward
While the potential of BNT116 is enormous, the road to its widespread application is still long and requires rigorous testing and evaluation. The success of the clinical trials will depend on many factors, including the vaccine’s ability to generate a strong immune response without causing adverse side effects. Moreover, scaling up production and ensuring the vaccine’s affordability and accessibility to patients worldwide will be crucial for its impact.
Experts in the field, including those from the World Health Organization (WHO) and the American Cancer Society (ACS), caution that while mRNA vaccines for cancer are promising, further research is needed to fully understand their long-term effectiveness and safety. However, the progress made by BioNTech is a step in the right direction and offers hope for millions of patients around the world.
Conclusion: A New Era in Cancer Care
The approval of the BNT116 vaccine for lung cancer represents a game-changing medical breakthrough. By utilizing the same powerful mRNA technology that was used to develop the COVID-19 vaccine, researchers are now working to combat one of the most deadly cancers in the world. With clinical trials now underway in multiple countries, the path to widespread application is closer than ever. If successful, the BNT116 vaccine could lead to a revolution in cancer treatment, providing more personalized, effective, and less invasive therapies for cancer patients globally.
Sources and Further Reading
-
BioNTech – Official announcements and ongoing research updates on mRNA cancer vaccines. BioNTech Official Website
-
World Health Organization (WHO) – Global cancer treatment strategies and immunotherapy research. WHO Website
-
American Cancer Society (ACS) – Cancer immunotherapy and mRNA vaccine developments. ACS Website
News in the same category


What It Means If Your Fingers Turn White When It’s Cold

Backpack Adventures Helping NYC Shelter Dogs Get Adopted

Unlock the Secret to Reducing Anxiety: How Placing Cold Under Your Arms Can Lower Heart Rate Instantly

Finland’s Youngest Prime Minister Opens Discussion On Shorter Working Week
Revolutionary Discovery: Tumor Microbiome Could Unlock New Frontiers in Cancer Diagnosis and Treatment!

CDC Ends All Monkey Testing in Its Laboratories

Six-Year-Old Returns to School to a Standing Ovation After Beating Leukemia

Baby penguins dive off 50-foot cliff in 1st-of-its-kind footage from National Geographic

Solar-Powered Laundry Huts in New Zealand Offer Homeless Families Dignity and Clean Clothes

Surprising Benefits of Sitting Facing Forward on the Toilet

Vanity Items Became Collectible Art

California to Require Seat-Belt Fit Test for Kids and Teens Before Switching from Booster Seats Starting 2027

Shaquille O'Neal’s Accidental Investment in Google Turned into a Fortune

UC San Francisco Researchers Discover Method to Transform White Fat into Calorie-Burning Beige Fat, Offering New Hope for Obesity Treatment

Japan Launches Groundbreaking Human Trial for Tooth Regeneration—Could We Soon Regrow Our Own Teeth?

Dolphins Join Elite Group of Animals with Self-Recognition, Challenging Our Understanding of Animal Intelligence

12 Signs You Have a Spiritual Gift (Even If You Don’t Realize It Yet)
News Post

The Hidden Psychology Behind Frequent Workout Posts

Thirteen Year Old Boy Becomes First Person Cured of Once Untreatable Brain Cancer

What It Means If Your Fingers Turn White When It’s Cold

Backpack Adventures Helping NYC Shelter Dogs Get Adopted

The Hidden Deficiency Ruining Your Eyesight (And How to Fix It)

Unlock the Secret to Reducing Anxiety: How Placing Cold Under Your Arms Can Lower Heart Rate Instantly

Finland’s Youngest Prime Minister Opens Discussion On Shorter Working Week

Revolutionary Discovery: Tumor Microbiome Could Unlock New Frontiers in Cancer Diagnosis and Treatment!
Scientists Trigger Cancer Cells to Destroy Themselves From the Inside Out

CDC Ends All Monkey Testing in Its Laboratories
Understanding Tinnitus Causes and Impact

🔪 What’s That Strange Toothy Part on Kitchen Scissors For?

Six-Year-Old Returns to School to a Standing Ovation After Beating Leukemia
One anti-inflammatory immunity shot works almost instantly
These 5 visible clues reveal insulin resistance long before blood tests

Baby penguins dive off 50-foot cliff in 1st-of-its-kind footage from National Geographic
10 vitamins and minerals you should never take — and why

Solar-Powered Laundry Huts in New Zealand Offer Homeless Families Dignity and Clean Clothes

Surprising Benefits of Sitting Facing Forward on the Toilet
