
Dual “Don’t-Eat-Me” Signals: A New Paradigm in Cancer Immune Evasion
Dual “Don’t-Eat-Me” Signals: A New Paradigm in Cancer Immune Evasion
The immune system plays a critical role in identifying and eliminating cancer cells, yet many tumors manage to survive and progress by actively suppressing immune responses. Among the key immune cells involved in early tumor clearance are macrophages, which normally engulf and destroy abnormal cells through a process known as phagocytosis. For years, cancer research has focused on understanding how tumors evade macrophage-mediated destruction. A major breakthrough came with the discovery of CD47, a surface protein that sends a powerful “don’t-eat-me” signal to macrophages. However, new research from Stanford University has revealed that cancer cells rely on not one, but two independent inhibitory signals to avoid immune elimination, fundamentally reshaping our understanding of tumor immune evasion.
In a study published in Nature Immunology, Stanford scientists demonstrated that tumors exploit both CD47 and major histocompatibility complex class I (MHC class I) molecules to suppress macrophage activity. CD47 was already well known as an immune checkpoint molecule that binds to the macrophage receptor SIRPα, effectively telling macrophages to stand down. Blocking CD47 with monoclonal antibodies has shown promising anti-tumor effects and is already being tested in human clinical trials. Yet, clinical responses to CD47 blockade alone have been variable, suggesting that tumors possess additional layers of protection.
The Stanford team uncovered this missing layer by identifying MHC class I as a second, distinct “don’t-eat-me” signal. While MHC class I is traditionally understood as a molecule that helps immune cells recognize “self” and avoid attacking healthy tissue, the researchers found that tumor-expressed MHC class I binds to an inhibitory receptor on macrophages called LILRB1. Engagement of LILRB1 sends a suppressive signal that directly inhibits the macrophage’s ability to engulf and destroy cancer cells. In effect, even when CD47 is blocked, the MHC class I–LILRB1 interaction can continue to restrain macrophage function.
Crucially, the study showed that disabling both pathways simultaneously produced dramatic anti-tumor effects. In mouse models, combined blockade of CD47 and LILRB1 led to rapid infiltration of tumors by immune cells, significant tumor shrinkage, and far more efficient tumor clearance. This dual inhibition effectively stripped cancer cells of their immune camouflage, allowing macrophages to recognize and eliminate them. The results suggest that many tumors survive not because one immune checkpoint is active, but because redundant, overlapping inhibitory systems provide a backup when one pathway is blocked.
These findings have important implications for cancer immunotherapy. Much like the success of combination checkpoint blockade in T-cell–based therapies (such as targeting PD-1 and CTLA-4 together), the study indicates that macrophage-directed therapies may also require combination strategies to achieve maximal efficacy. Targeting both CD47 and the MHC class I–LILRB1 axis could transform macrophages from passive bystanders into potent anti-tumor effectors, particularly in cancers that are resistant to current immunotherapies.
In conclusion, the Stanford study published in Nature Immunology reveals that cancer cells employ at least two independent “don’t-eat-me” signals to evade macrophage-mediated destruction (Nature Immunology, year of publication). By uncovering the cooperative roles of CD47 and MHC class I in suppressing phagocytosis, this research highlights a powerful new therapeutic strategy: disabling multiple immune escape mechanisms at once. Such an approach may significantly enhance the immune system’s ability to recognize, attack, and ultimately eliminate cancer.
News in the same category

Sodium Bicarbonate and Immune Regulation: Evidence for an Anti-Inflammatory Mechanism
Oleocanthal from Extra-Virgin Olive Oil: A Multi-Targeted Anti-Inflammatory and Anti-Cancer Compound

Fenbendazole and Unexpected Cancer Remission: Insights from a 2024 Case Report
Quercetin as an Exercise Mimetic: Enhancing Mitochondrial Biogenesis and Physical Performance

Cinnamon and Neuroprotection: Evidence for Anti-Alzheimer’s Mechanisms

High-Dose Thiamine and Cancer Cell Metabolism: Evidence from Experimental Cell-Line Studies
How to Improve Blood Circulation Naturally (Research Based)

The Most Effective Ways to Naturally Get Rid of Clogged Ears
Get Rid of Throat Mucus Faster With These Home Treatments (Evidence Based)

The Most Likely Symptoms of a Gallbladder Problem (Don’t Ignore Them)
Why Hearing Running Water Makes You Suddenly Need to Pee

The strange phenomenon of sleep paralysis: When the body stops listening

Surprising Health Benefits of Purslane (Portulaca oleracea)

Ageratum Conyzoides: A Valuable Herb with Many Health Benefits

The man died of colorectal cancer, his family broke down in tears: "He ate healthily but liked to do three things."

Women Who Regularly Eat These 4 Foods Can Prevent Premature Graying, Keeping Hair Dark and Shiny Even at 60

The Root that Helps the Liver Detoxify and Effectively Reduce Inflammation
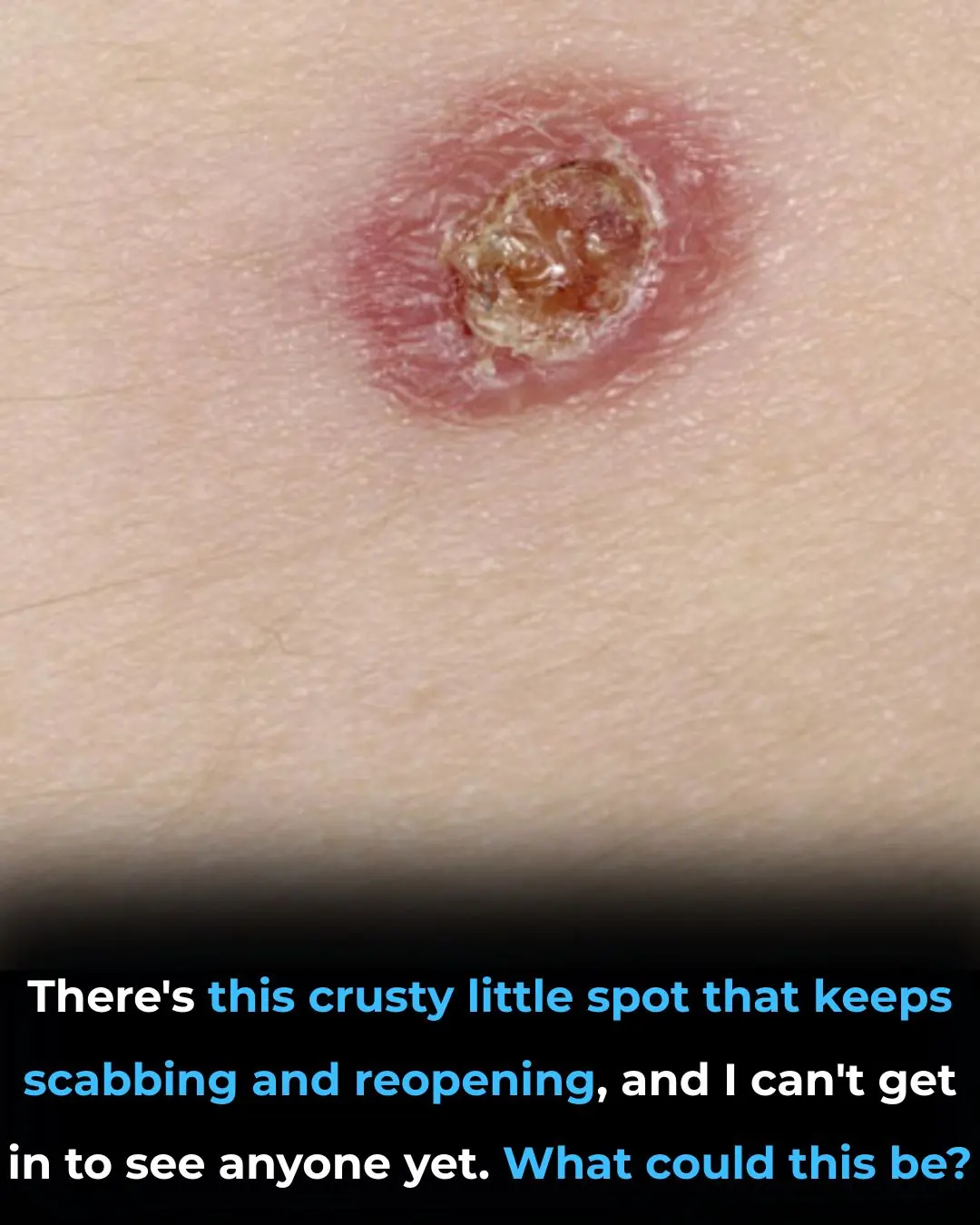
There’s this crusty little spot that keeps scabbing and reopening, and I can’t get in to see anyone yet. What could this be?
News Post

Nighttime Olfactory Enrichment and Cognitive Enhancement in Older Adults

Sodium Bicarbonate and Immune Regulation: Evidence for an Anti-Inflammatory Mechanism

Oleocanthal from Extra-Virgin Olive Oil: A Multi-Targeted Anti-Inflammatory and Anti-Cancer Compound

Fenbendazole and Unexpected Cancer Remission: Insights from a 2024 Case Report

Quercetin as an Exercise Mimetic: Enhancing Mitochondrial Biogenesis and Physical Performance

Cinnamon and Neuroprotection: Evidence for Anti-Alzheimer’s Mechanisms

High-Dose Thiamine and Cancer Cell Metabolism: Evidence from Experimental Cell-Line Studies
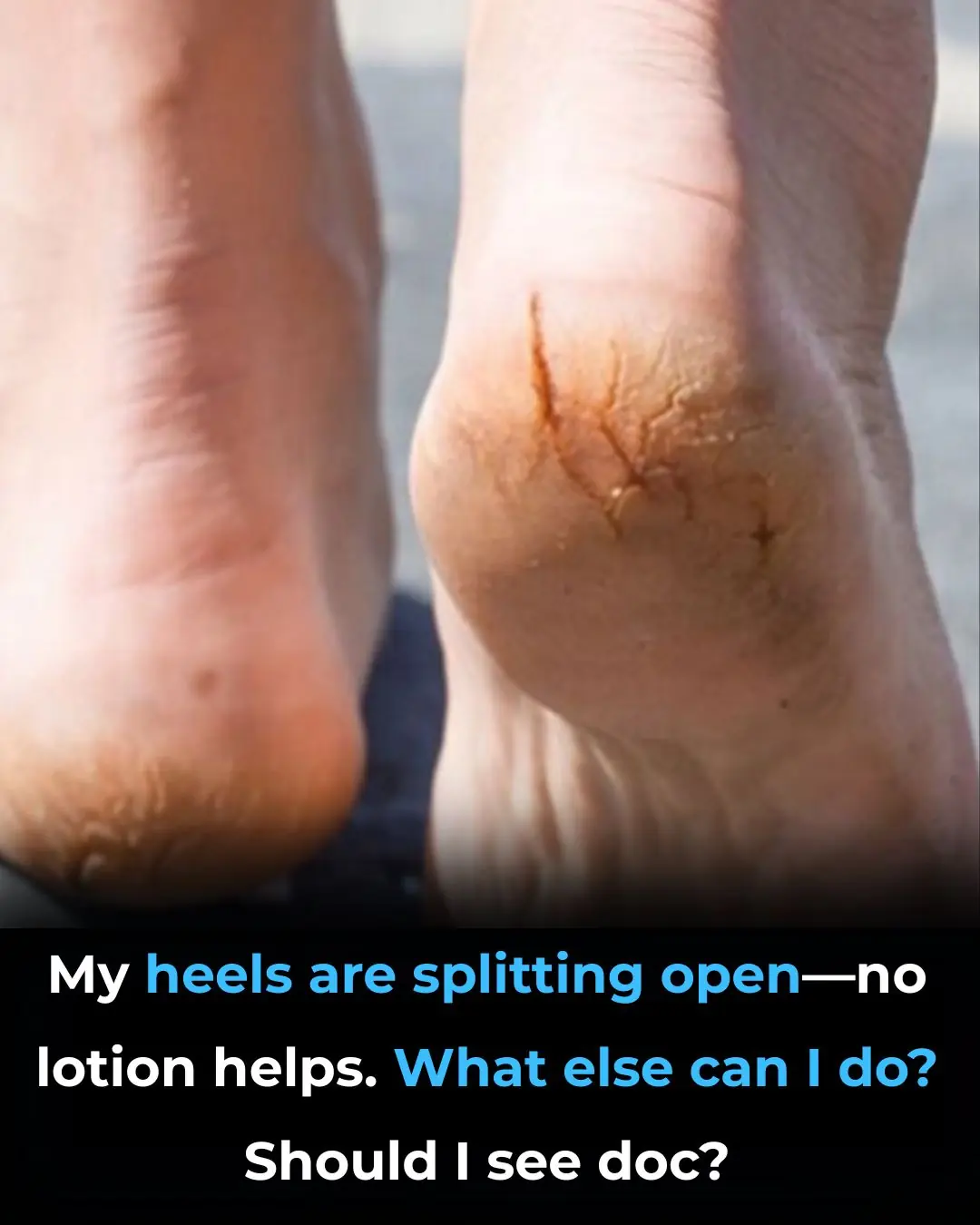
My heels are splitting open—no lotion helps. What else can I do? Should I see doc?
My aunt swears by this trick to reduce the look of thinning eyebrows that takes almost 0 work. Here's how it works
These purple veins appeared out of nowhere, and my doctor appointment is still weeks away. Should I worry?

Brown flat spots keep showing up on the back of my hands. Doctor appt is forever away. What should I do?

How to Improve Blood Circulation Naturally (Research Based)

The Most Effective Ways to Naturally Get Rid of Clogged Ears

Get Rid of Throat Mucus Faster With These Home Treatments (Evidence Based)

The Most Likely Symptoms of a Gallbladder Problem (Don’t Ignore Them)

If A Woman Says These 6 Things Regularly, She’s Way Smarter Than You Even Realize

11 Clever Phrases Smart People Use to End Pointless Arguments

Mopping the floor with this ingredient will make it sparkling clean, like new, and dust-free for a whole week.
