
Fecal Microbiota Transplantation and Its Potential Role in Severe Autism Spectrum Disorder
Fecal Microbiota Transplantation and Its Potential Role in Severe Autism Spectrum Disorder
Autism spectrum disorder (ASD) is a complex neurodevelopmental condition characterized by impairments in social communication, restricted interests, and repetitive behaviors. A substantial proportion of children with ASD also suffer from chronic gastrointestinal (GI) dysfunction, including constipation, diarrhea, abdominal pain, and intestinal inflammation. Growing evidence suggests a bidirectional relationship between the gut microbiota and the brain, commonly referred to as the gut–brain axis. Within this context, emerging therapeutic strategies aimed at modifying the gut microbiome have attracted increasing scientific attention. A 2023 case report published in Frontiers in Psychiatry provides a compelling illustration of this approach through the use of fecal microbiota transplantation (FMT) in a child with severe ASD and longstanding GI symptoms.
The case involved a 7-year-old girl diagnosed with severe ASD who had experienced minimal improvement despite years of intensive behavioral therapy. In addition to profound language impairment and social deficits, she suffered from chronic gastrointestinal problems accompanied by inflammatory changes in the ileum and rectum. Prior to FMT, the patient underwent a 14-day course of oral vancomycin to reduce existing gut bacteria, a preparatory step intended to facilitate successful colonization by donor microbiota. Subsequently, she received five FMT treatments over a three-month period using stool from a healthy, age-matched donor. The initial transplant was delivered via colonoscopy into the terminal ileum, while the remaining doses were administered by enema.
Following the intervention, the child demonstrated marked and multifaceted improvements. Core ASD symptoms showed clear positive changes, including improved eye contact, reduced stereotyped behaviors, increased social interest, and greater emotional responsiveness. Notably, her language abilities improved substantially: she progressed from being essentially nonverbal to using words and simple sentences, expressing needs, and verbally communicating affection, such as calling “Mom” and saying “I love you.” These behavioral and communicative gains were accompanied by rapid resolution of gastrointestinal symptoms. Follow-up endoscopic examinations revealed substantial healing of the previously inflamed intestinal mucosa.
Standardized autism assessment tools—including the Childhood Autism Rating Scale (CARS), Autism Treatment Evaluation Checklist (ATEC), Aberrant Behavior Checklist (ABC), and Social Responsiveness Scale (SRS)—all demonstrated consistent and clinically meaningful reductions in symptom severity. These objective measures strengthened the observation that improvements were not limited to subjective reports but were reflected across validated clinical instruments.
Microbiome analyses provided further insight into potential mechanisms underlying these changes. Before treatment, the child’s gut microbiota exhibited low diversity, a feature commonly reported in individuals with ASD. After FMT, her microbial profile shifted markedly toward that of the donor, with increased abundance of genera such as Bacteroides and Ruminococcus. There was also evidence of enhanced capacity for short-chain fatty acid production and greater overall microbial diversity—factors associated with improved gut barrier integrity, reduced inflammation, and modulation of neuroimmune signaling.
In conclusion, this case report published in Frontiers in Psychiatry highlights the potential of fecal microbiota transplantation as a novel therapeutic avenue for select children with ASD and comorbid gastrointestinal dysfunction (Frontiers in Psychiatry, 2023). While the findings are limited by the single-case design and cannot establish causality, the magnitude and consistency of improvements across behavioral, gastrointestinal, and microbiological domains are noteworthy. The authors appropriately emphasize the need for larger, controlled clinical trials to confirm efficacy, assess safety, and clarify the biological mechanisms linking gut microbiota alterations to neurodevelopmental outcomes.
News in the same category

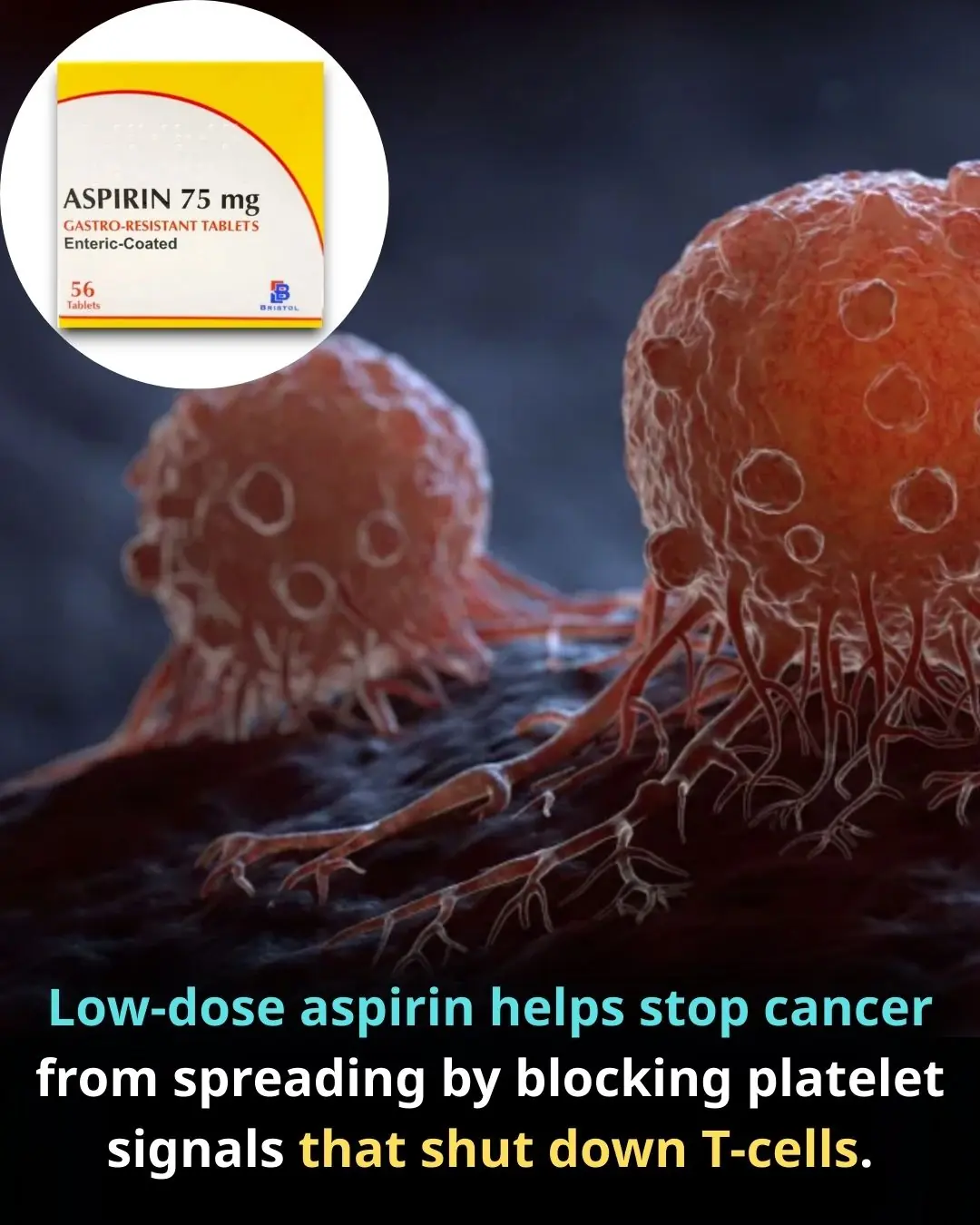
Aspirin as an Immune-Modulating Agent in the Suppression of Cancer Metastasis

Gum disease bacteria found in alzheimer’s brains

Raw Carrots and Their Impact on Cholesterol and Colon Function

Breakthrough in Pancreatic Cancer Immunotherapy

Blue Blood in the Ocean: How Horseshoe Crabs Help Protect Human Health

You were raised by emotionally manipulative parents if you heard these 8 phrases as a child

The reason some seniors decline after moving to nursing homes

Sauna Bathing and Long-Term Cardiovascular Health: Evidence from a Finnish Cohort Study

The Role of Dietary Cysteine in Intestinal Repair and Regeneration
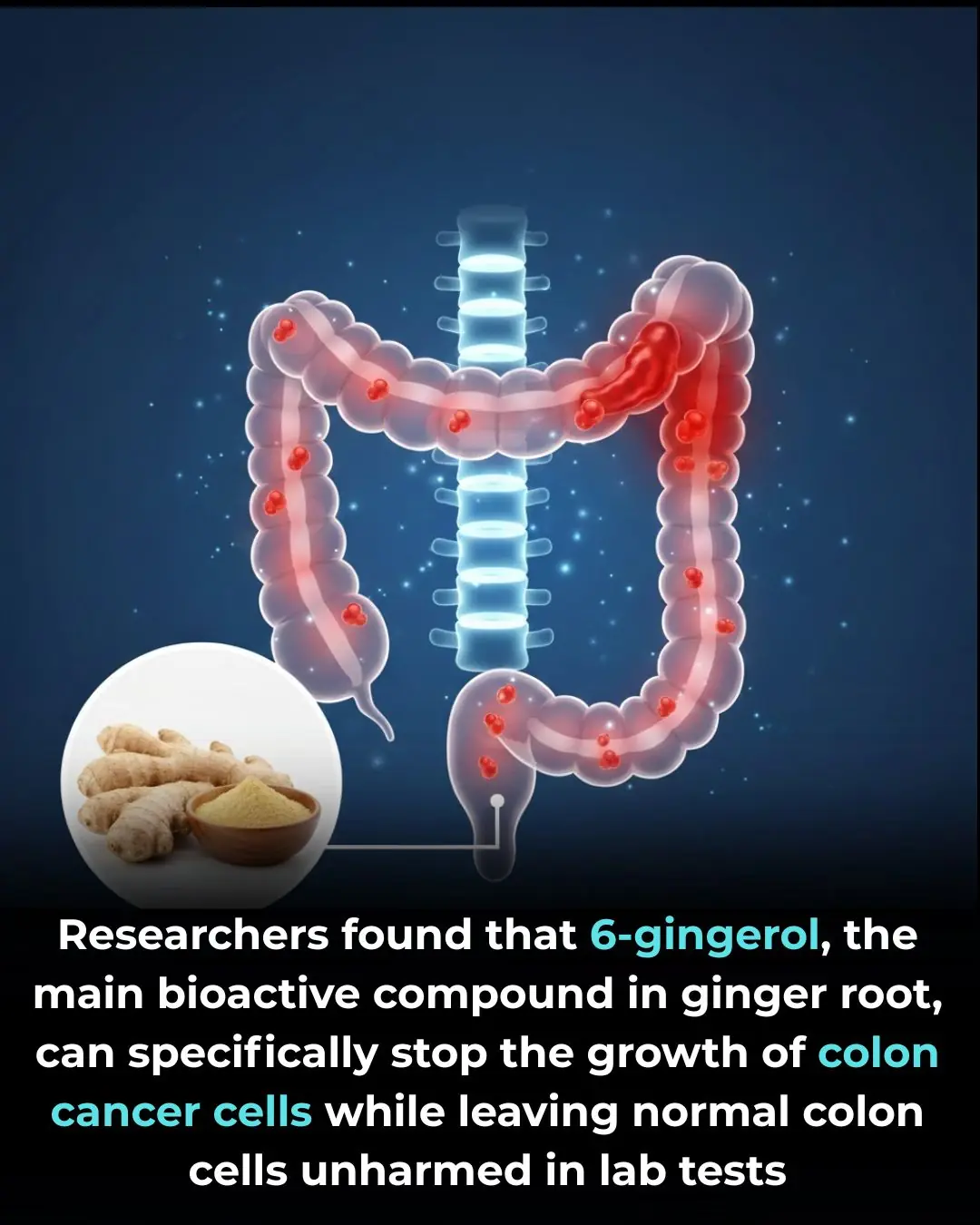
Researchers found that 6-gingerol, the main bioactive compound in ginger root, can specifically stop the growth of colon cancer cells while leaving normal colon cells unharmed in lab tests
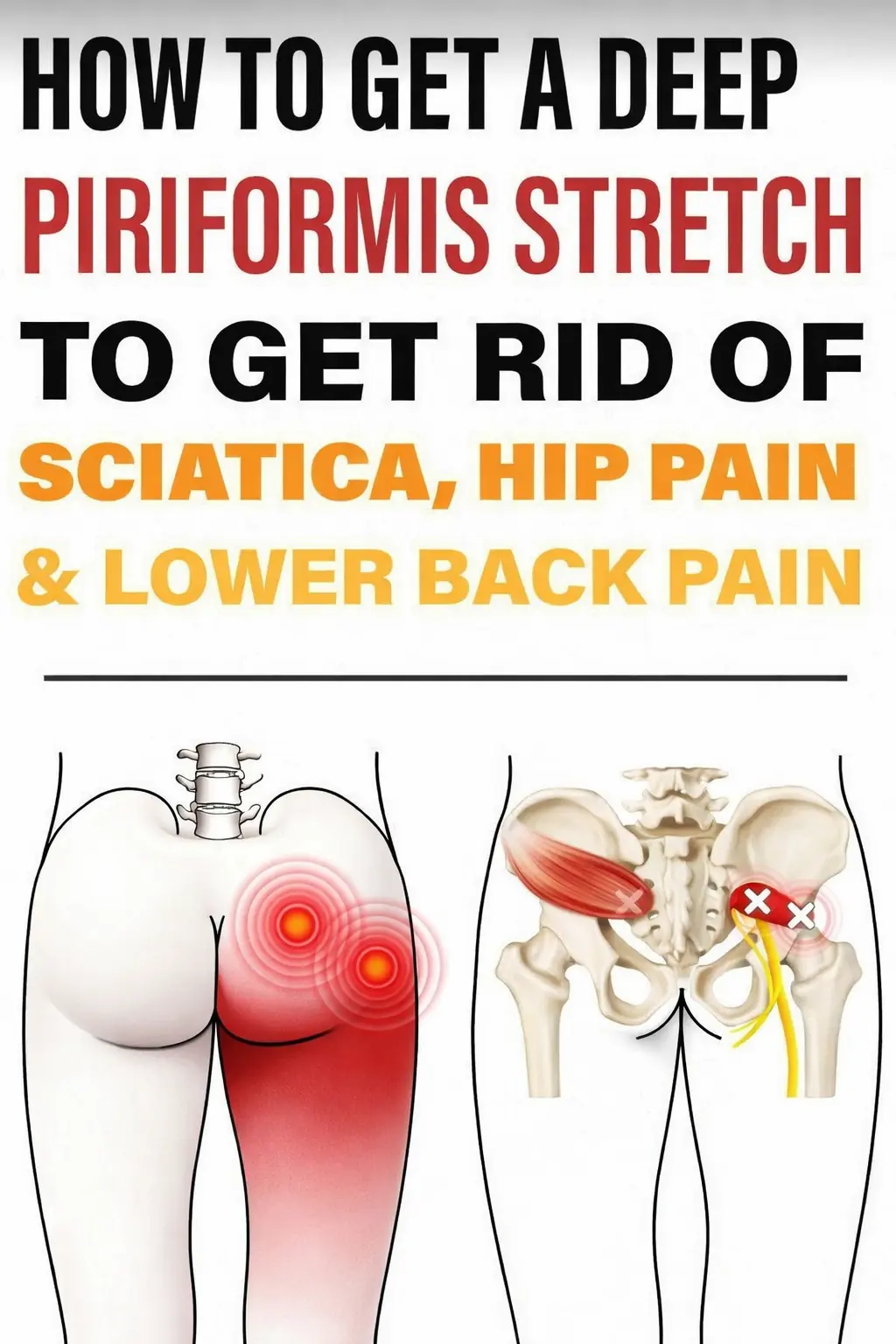
Powerful Piriformis Stretch to Soothe Sciatic, Hip, and Lower Back Pain

Napping During The Day Seriously Affects Brain Aging

Waking at 3 AM every night? 4 hidden causes

Waking at 3 AM every night? 4 hidden causes
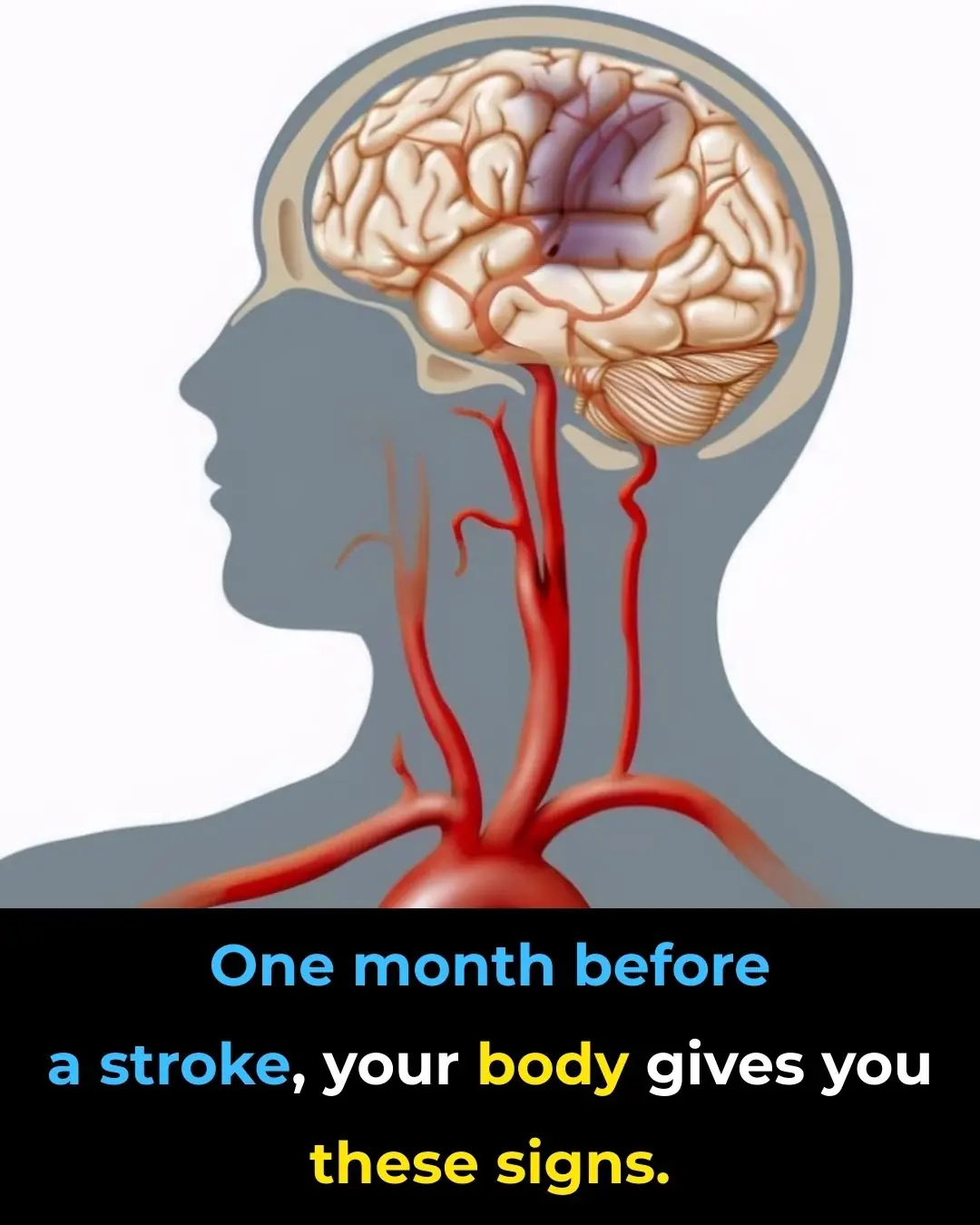
Sudden confusion or difficulty speaking: when it’s more than just fatigue

The Four Best Times to Drink Coffee for Maximum Health Benefits

7 Tips to Give Your Evening Routine a Refresh

Your Butt May Reveal Your Diabetes Risk
News Post

Psilocybin and the Biology of Aging: Emerging Experimental Evidence

Aspirin as an Immune-Modulating Agent in the Suppression of Cancer Metastasis

Gum disease bacteria found in alzheimer’s brains
4 tips for cleaning and polishing leather shoes at home without shoe polish, using ingredients readily available in every kitchen.
7 ways to remove refrigerator odors using safe, natural ingredients.

Raw Carrots and Their Impact on Cholesterol and Colon Function

Breakthrough in Pancreatic Cancer Immunotherapy

How to grow lemons in pots for abundant fruit all year round, more than enough for the whole family to eat

Regularly using these three types of cooking oil can lead to liver cancer without you even realizing it.

6 tips for using beer as a hair mask or shampoo to make hair shiny, dark, and reduce hair loss

When rendering pork fat, don't just put it directly into the pan. Adding this extra step ensures that every batch of pork fat is perfectly white and won't mold even after a long time.
Scientists Crack an “Impossible” Cancer Target With a Promising New Drug

Blue Blood in the Ocean: How Horseshoe Crabs Help Protect Human Health

Using an electric kettle to boil water: 9 out of 10 households make this mistake, so remind your family members to correct it soon

4 "cancer culprits" lurking in your home, many people are exposed to daily without knowing it
Three "strange" red spots on the body are actually signs of cancer that very few people notice
5 foot changes that signal liver "exhaustion," a sign that you may have had liver cancer for a long time.

10 types of fruits and vegetables you should never put in the refrigerator; many people don't know this and end up doing it wrong, ruining the taste and causing them to spoil quickly.
