
Gum disease bacteria found in alzheimer’s brains
Chronic Oral Infection as a Potential Driver of Alzheimer’s Disease Pathology
Alzheimer’s disease is the most common cause of dementia worldwide and is characterized by progressive cognitive decline, neurodegeneration, amyloid-β plaque accumulation, and tau pathology. Despite decades of research, its underlying causes remain incompletely understood, and effective disease-modifying therapies are still lacking. In recent years, increasing attention has been paid to the possible role of chronic infections and inflammation in neurodegenerative disorders. A landmark 2019 study published in Science Advances provided compelling experimental evidence linking a common oral pathogen to the core pathological features of Alzheimer’s disease.
The study, led by Stephen Dominy and colleagues at the biotechnology company Cortexyme, focused on Porphyromonas gingivalis, a bacterium strongly associated with chronic periodontitis (gum disease). Periodontitis is a widespread inflammatory condition, particularly prevalent in older adults, and has long been epidemiologically associated with cognitive decline. However, prior to this research, it remained unclear whether oral bacteria played a causal role in Alzheimer’s disease or were merely correlated with it. Dominy and his team addressed this question by examining postmortem human brain tissue and conducting mechanistic experiments in animal models.
Analysis of brain samples from individuals who had died with Alzheimer’s disease revealed the presence of P. gingivalis DNA and its toxic proteolytic enzymes, known as gingipains, directly within the brain. Importantly, gingipain levels closely correlated with the severity of tau tangles and markers of neurodegeneration. This association suggested that the bacterium was not an incidental finding but potentially involved in driving disease processes. Gingipains are known to damage host tissues and disrupt immune responses, making them biologically plausible contributors to neuronal injury.
To strengthen the causal argument, the researchers conducted experiments in mice. When mice were orally infected with P. gingivalis, the bacterium entered the bloodstream, crossed into the brain, and triggered a significant increase in amyloid-β (Aβ1–42), a peptide central to Alzheimer’s pathology. Notably, amyloid accumulation is often considered an early and initiating event in the disease. Even more compelling, pharmacological inhibition of gingipains reduced bacterial load in the brain and significantly lowered amyloid production. These findings demonstrated that blocking a specific bacterial virulence factor could directly influence hallmark features of Alzheimer’s disease.
The implications of this research are substantial. Rather than viewing Alzheimer’s disease solely as a disorder of abnormal protein aggregation, this study supports the hypothesis that chronic infection and inflammation—originating outside the brain—may actively contribute to neurodegeneration. If oral pathogens such as P. gingivalis can initiate or accelerate Alzheimer’s pathology, then preventive strategies targeting oral health or antimicrobial treatments may represent novel therapeutic avenues. This perspective also reinforces the importance of oral hygiene and periodontal care as potential components of long-term brain health.
In conclusion, the 2019 study published in Science Advances provides direct experimental evidence that chronic oral infection with Porphyromonas gingivalis may play a causal role in Alzheimer’s disease rather than merely coexisting with it (Science Advances, 2019). While further clinical studies are needed to confirm these findings in larger populations and to evaluate therapeutic interventions, this research represents a significant shift in how Alzheimer’s disease etiology may be understood, integrating infectious biology into the framework of neurodegenerative disease research.
News in the same category

Aspirin as an Immune-Modulating Agent in the Suppression of Cancer Metastasis

Fecal Microbiota Transplantation and Its Potential Role in Severe Autism Spectrum Disorder

Raw Carrots and Their Impact on Cholesterol and Colon Function

Breakthrough in Pancreatic Cancer Immunotherapy

Blue Blood in the Ocean: How Horseshoe Crabs Help Protect Human Health

You were raised by emotionally manipulative parents if you heard these 8 phrases as a child

The reason some seniors decline after moving to nursing homes

Sauna Bathing and Long-Term Cardiovascular Health: Evidence from a Finnish Cohort Study

The Role of Dietary Cysteine in Intestinal Repair and Regeneration
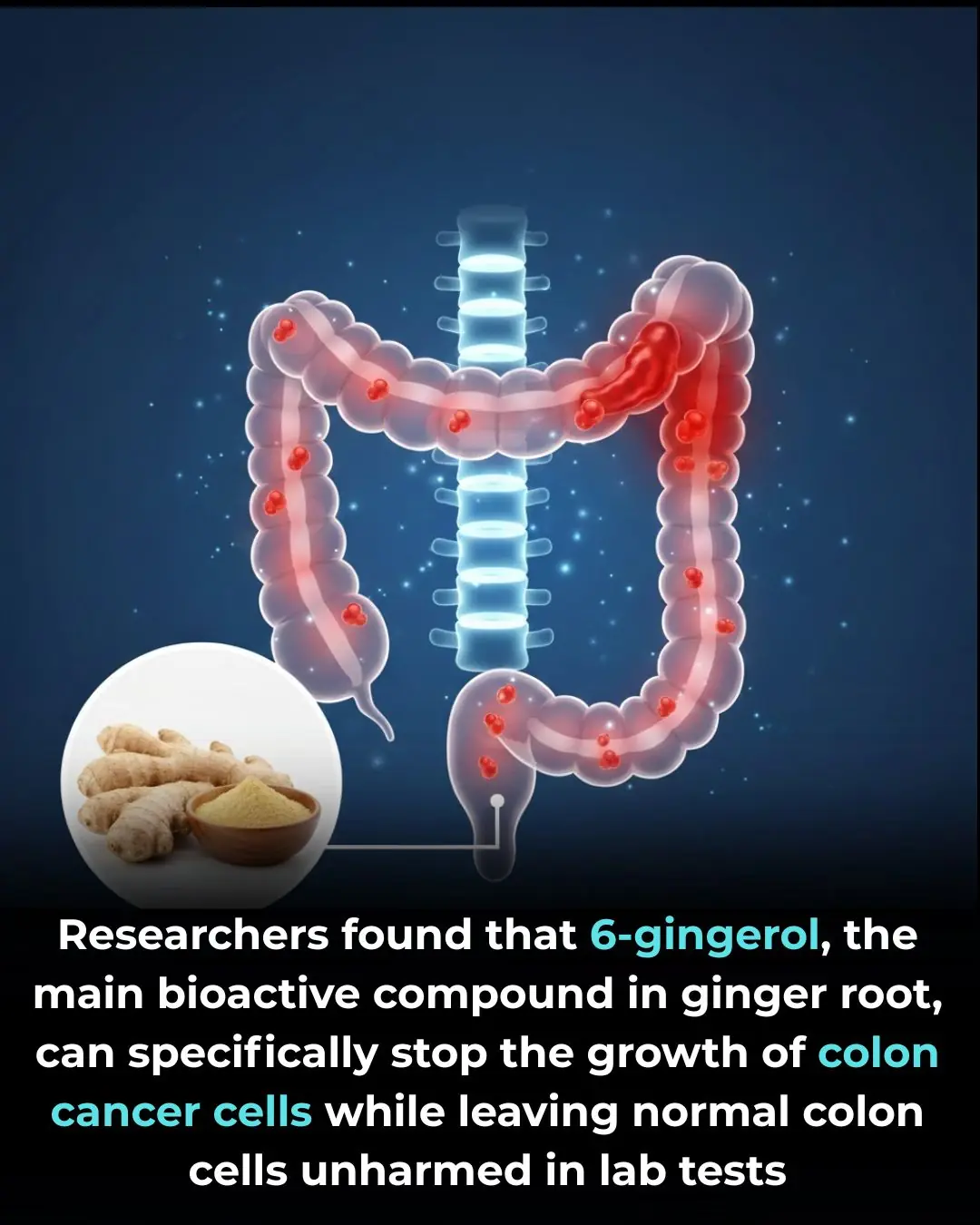
Researchers found that 6-gingerol, the main bioactive compound in ginger root, can specifically stop the growth of colon cancer cells while leaving normal colon cells unharmed in lab tests
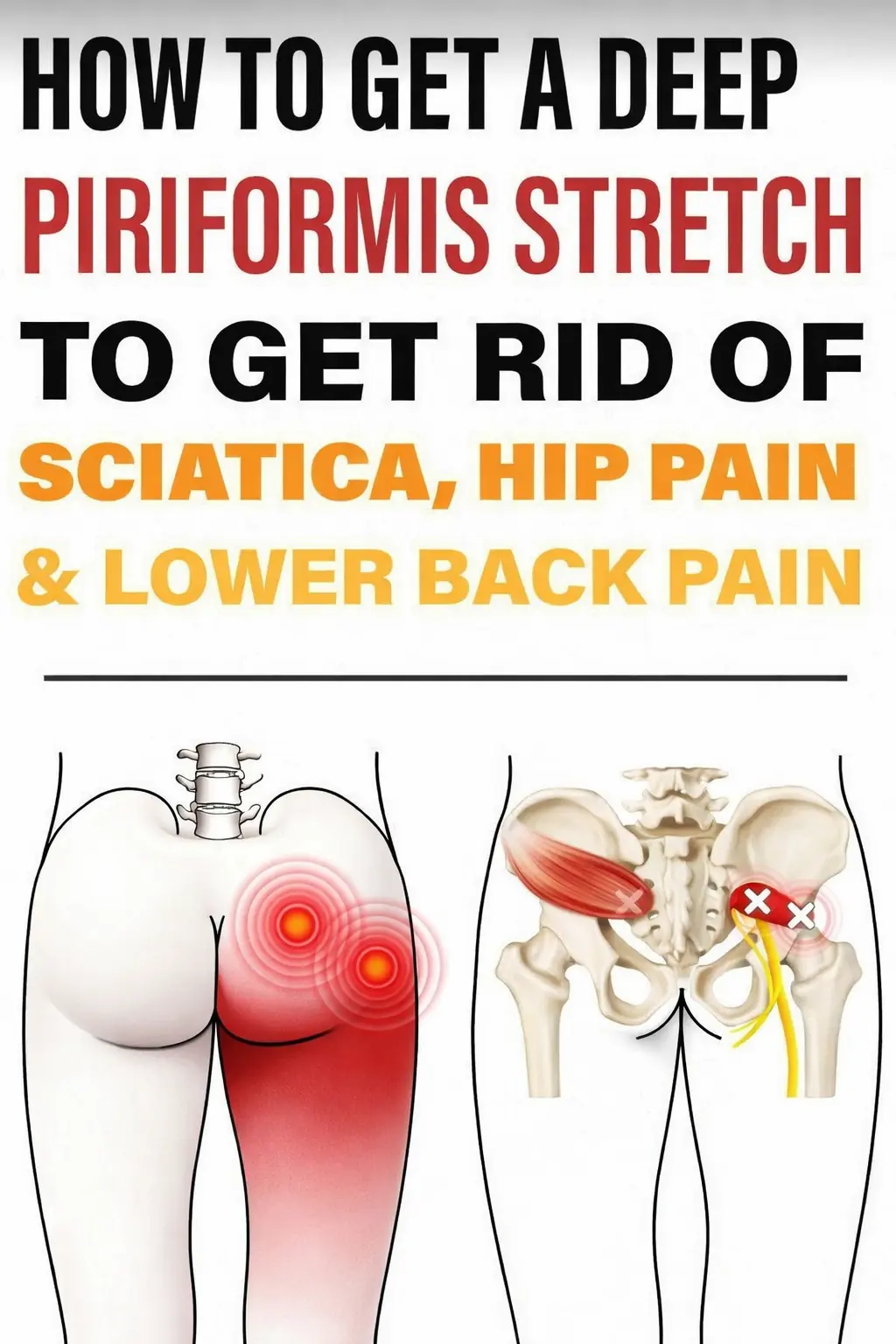
Powerful Piriformis Stretch to Soothe Sciatic, Hip, and Lower Back Pain

Napping During The Day Seriously Affects Brain Aging

Waking at 3 AM every night? 4 hidden causes

Waking at 3 AM every night? 4 hidden causes
Sudden confusion or difficulty speaking: when it’s more than just fatigue

The Four Best Times to Drink Coffee for Maximum Health Benefits

7 Tips to Give Your Evening Routine a Refresh

Your Butt May Reveal Your Diabetes Risk
News Post

Psilocybin and the Biology of Aging: Emerging Experimental Evidence

Aspirin as an Immune-Modulating Agent in the Suppression of Cancer Metastasis

Fecal Microbiota Transplantation and Its Potential Role in Severe Autism Spectrum Disorder
4 tips for cleaning and polishing leather shoes at home without shoe polish, using ingredients readily available in every kitchen.
7 ways to remove refrigerator odors using safe, natural ingredients.

Raw Carrots and Their Impact on Cholesterol and Colon Function

Breakthrough in Pancreatic Cancer Immunotherapy

How to grow lemons in pots for abundant fruit all year round, more than enough for the whole family to eat

Regularly using these three types of cooking oil can lead to liver cancer without you even realizing it.

6 tips for using beer as a hair mask or shampoo to make hair shiny, dark, and reduce hair loss

When rendering pork fat, don't just put it directly into the pan. Adding this extra step ensures that every batch of pork fat is perfectly white and won't mold even after a long time.
Scientists Crack an “Impossible” Cancer Target With a Promising New Drug

Blue Blood in the Ocean: How Horseshoe Crabs Help Protect Human Health

Using an electric kettle to boil water: 9 out of 10 households make this mistake, so remind your family members to correct it soon

4 "cancer culprits" lurking in your home, many people are exposed to daily without knowing it
Three "strange" red spots on the body are actually signs of cancer that very few people notice
5 foot changes that signal liver "exhaustion," a sign that you may have had liver cancer for a long time.

10 types of fruits and vegetables you should never put in the refrigerator; many people don't know this and end up doing it wrong, ruining the taste and causing them to spoil quickly.
