Regenerating True Articular Cartilage: A Breakthrough in Joint Repair
Regenerating True Articular Cartilage: A Breakthrough in Joint Repair
Damage to articular cartilage—the smooth, load-bearing tissue that covers the ends of bones in joints—is a central problem in osteoarthritis and traumatic joint injury. Unlike many other tissues, articular cartilage has very limited natural regenerative capacity. When it is damaged, the body typically produces fibrocartilage, a mechanically inferior substitute that lacks the durability and resilience of true articular cartilage. This limitation is a major reason why joint degeneration often progresses irreversibly, ultimately requiring joint replacement surgery. In this context, a recent study from Stanford researchers published in Nature Medicine represents a major advance in regenerative medicine by demonstrating a method to regenerate authentic articular cartilage rather than scar-like fibrocartilage.
The researchers focused on skeletal stem cells, a population of progenitor cells capable of giving rise to bone, cartilage, and other skeletal tissues. Their strategy was based on the insight that cartilage and bone formation share early developmental pathways. Instead of attempting to directly force stem cells to become cartilage, the team precisely guided the cells through the natural stages of skeletal development and then deliberately interrupted the process at the correct point. This level of temporal control proved to be the key to generating high-quality cartilage.
The process began with a controlled micro-injury to the joint, which activated resident skeletal stem cells. The researchers then administered bone morphogenetic protein 2 (BMP2), a well-known signaling molecule that initiates early bone formation. Importantly, BMP2 also stimulates the formation of a cartilage template, known as a cartilage callus, which normally precedes bone development. At this critical stage, the team intervened by blocking vascular endothelial growth factor (VEGF), a signal required for blood vessel invasion and the subsequent transition from cartilage to bone. By inhibiting VEGF, they effectively “froze” the cells in the cartilage phase, preventing ossification and allowing stable articular cartilage to form.
In mouse models, this approach produced cartilage that closely resembled native joint cartilage in both structure and function. Mechanical testing showed that the regenerated tissue had stiffness and load-bearing properties comparable to natural articular cartilage. Functionally, osteoarthritic mice treated with this method exhibited restored joint mobility and improved movement, indicating that the regenerated cartilage was not merely anatomically correct but also biologically and mechanically effective.
A particularly important aspect of the study was its relevance to human biology. When tissue containing human skeletal stem cells was transplanted into mice, it responded to the same sequence of signals in an identical manner. This demonstrated that the underlying regeneration pathway is conserved between mice and humans, significantly strengthening the translational potential of the findings. Rather than being a species-specific phenomenon, the mechanism appears to reflect a fundamental principle of skeletal tissue development.
The implications of this research are substantial. Current clinical approaches to cartilage damage, such as microfracture surgery or cartilage grafting, often result in fibrocartilage formation and only temporary symptom relief. In contrast, the strategy described in Nature Medicine offers a regenerative solution that restores the original tissue type. If successfully translated into humans, this approach could allow clinicians to repair joints at early stages of degeneration, potentially halting or even reversing the progression toward osteoarthritis and eliminating the need for joint replacement in many patients.
In conclusion, the Stanford study published in Nature Medicine demonstrates that true articular cartilage regeneration is achievable by precisely controlling skeletal stem cell fate. By initiating bone formation and then strategically halting it, researchers were able to recreate durable, functional cartilage that restores joint movement. This work lays critical groundwork for future regenerative therapies that address joint damage at its biological root, offering new hope for millions of patients suffering from degenerative joint disease (Nature Medicine, year of publication).
News in the same category


Daily Prune Consumption and Bone Health in Postmenopausal Women

Montmorency Tart Cherry Juice as an Adjunctive Therapy in Ulcerative Colitis
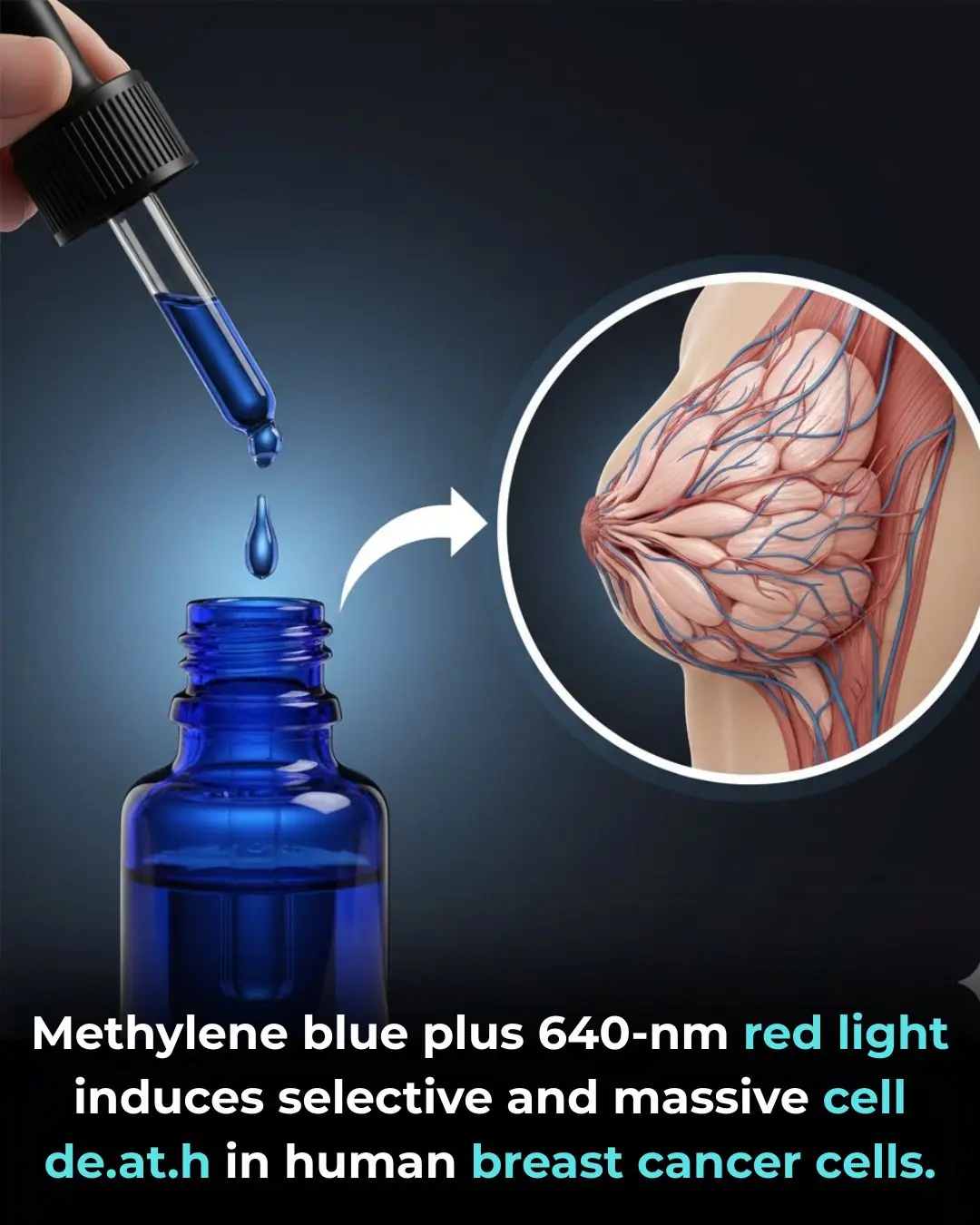
Methylene Blue–Based Photodynamic Therapy as a Selective Strategy Against Breast Cancer Cells

Deuterium-Depleted Water and Long-Term Survival in Cancer Patients: Evidence from a Hungarian Population-Based Analysis

Daily cocoa extract supplements lowered cardiovascular disease deaths by 27%

Ivermectin stopped aggressive cancer cells from moving and spreading in lab tests
The Best Natural Gout Treatments: Remove Uric Acid Crystallization To Prevent Gout And Joint Pain
Quickly Drain You Lymph System Using Theses Simple Techniques to Boost Immunity and Remove Toxins
The Top 20 Essential Oils to Relieve Pain and Inflammation (Research Based)

Headache Above or Behind the Left Eye: Causes and Treatments
Root Canals May Lower Risk of Heart Disease, Diabetes

Mother-to-Infant Microbiome Transmission: Beyond Bacteria to Genes
Vitamin C Supplementation and Its Targeted Impact on the Human Gut Microbiome

Sleep and Dementia Risk: What You Should Know

Warning: 4 things to avoid when napping to prevent illness

The Amazing Benefits of Guava Leaf Water That Few People Know

Are Vaccines Doing More Than Just Preventing Infection?
News Post
Australian HPV Vaccination Marks Cervical Cancer Milestone

My outdoor faucet suddenly froze and now I’m seeing water seeping indoors — what should I do before a plumber can come?

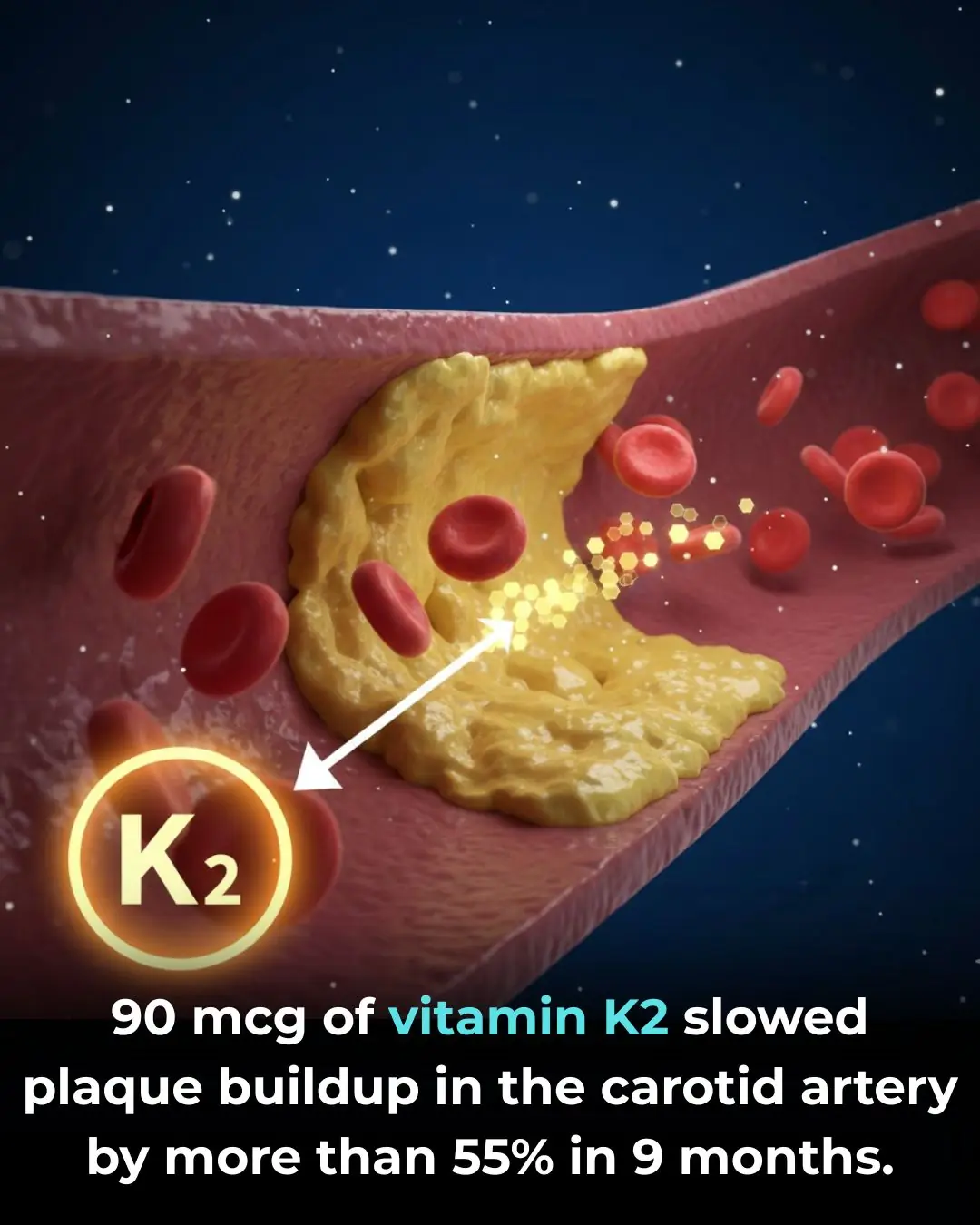
Vitamin K2 Supplementation and Vascular Health in Chronic Kidney Disease

Daily Prune Consumption and Bone Health in Postmenopausal Women

Montmorency Tart Cherry Juice as an Adjunctive Therapy in Ulcerative Colitis

Methylene Blue–Based Photodynamic Therapy as a Selective Strategy Against Breast Cancer Cells

Deuterium-Depleted Water and Long-Term Survival in Cancer Patients: Evidence from a Hungarian Population-Based Analysis

Daily cocoa extract supplements lowered cardiovascular disease deaths by 27%

Ivermectin stopped aggressive cancer cells from moving and spreading in lab tests
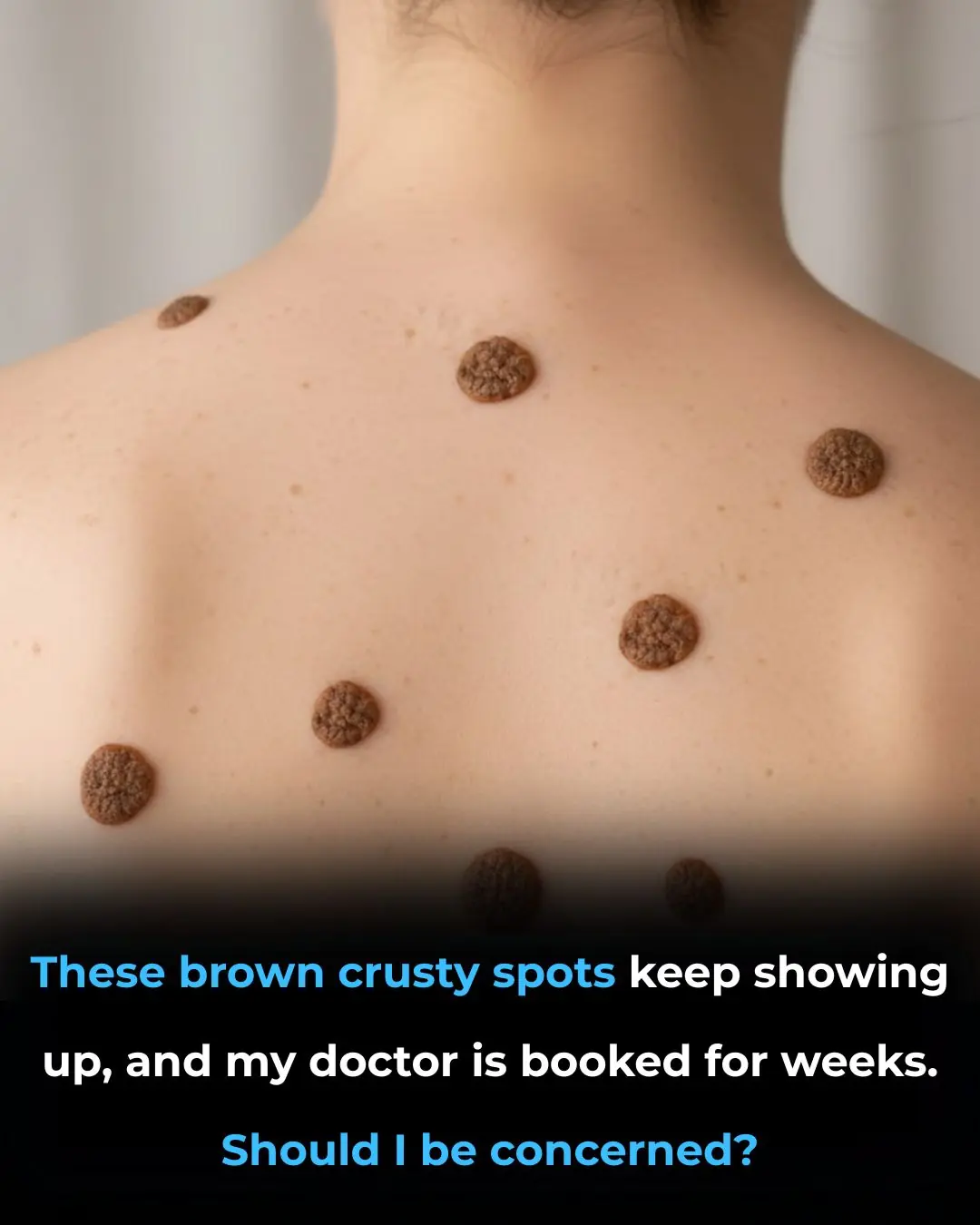
These brown crusty spots keep showing up, and my doctor is booked for weeks given Christmas. Should I be concerned?
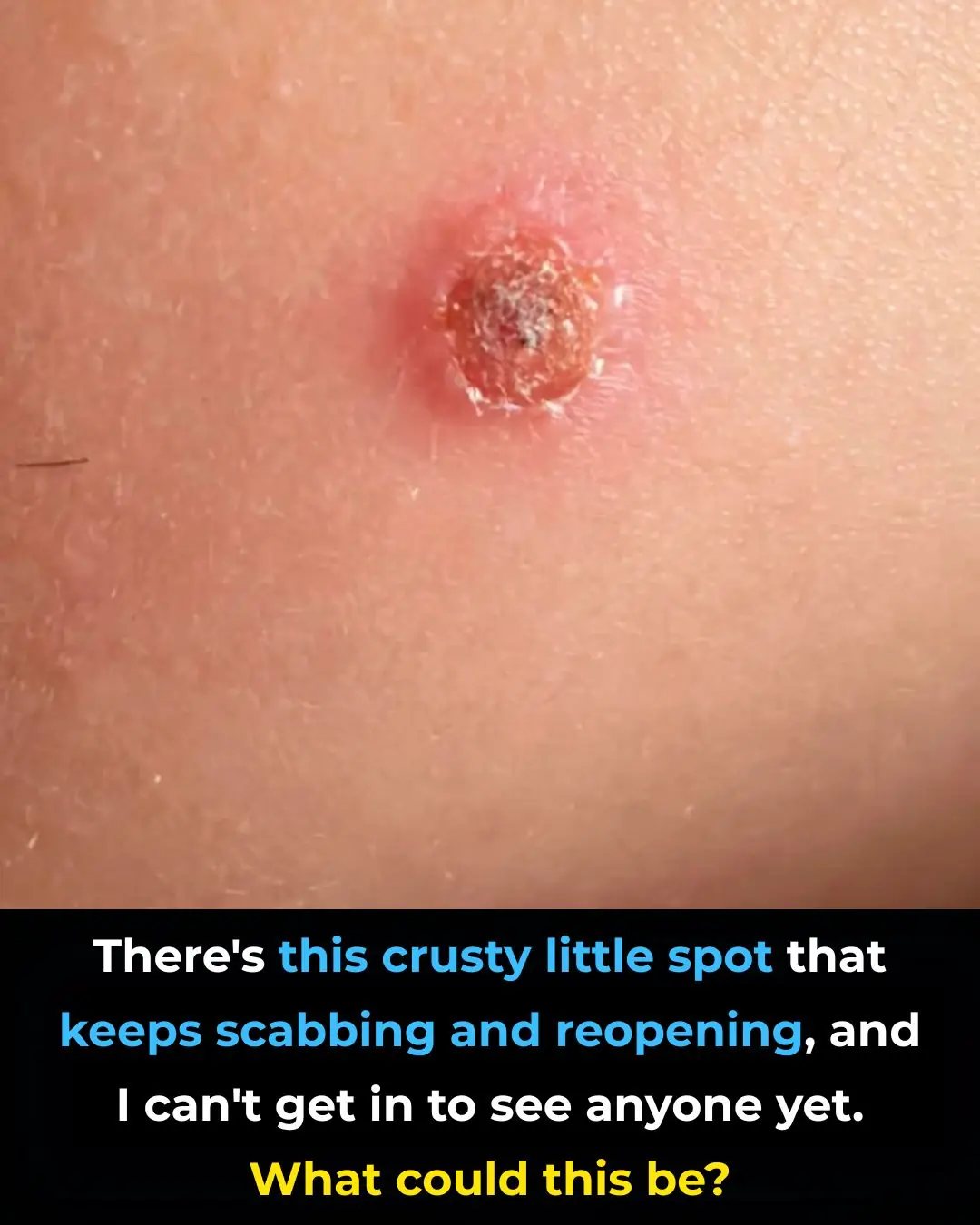
There’s this crusty little spot that keeps scabbing and reopening, and I can’t get in to see anyone yet. What could this be?

I had no clue about this
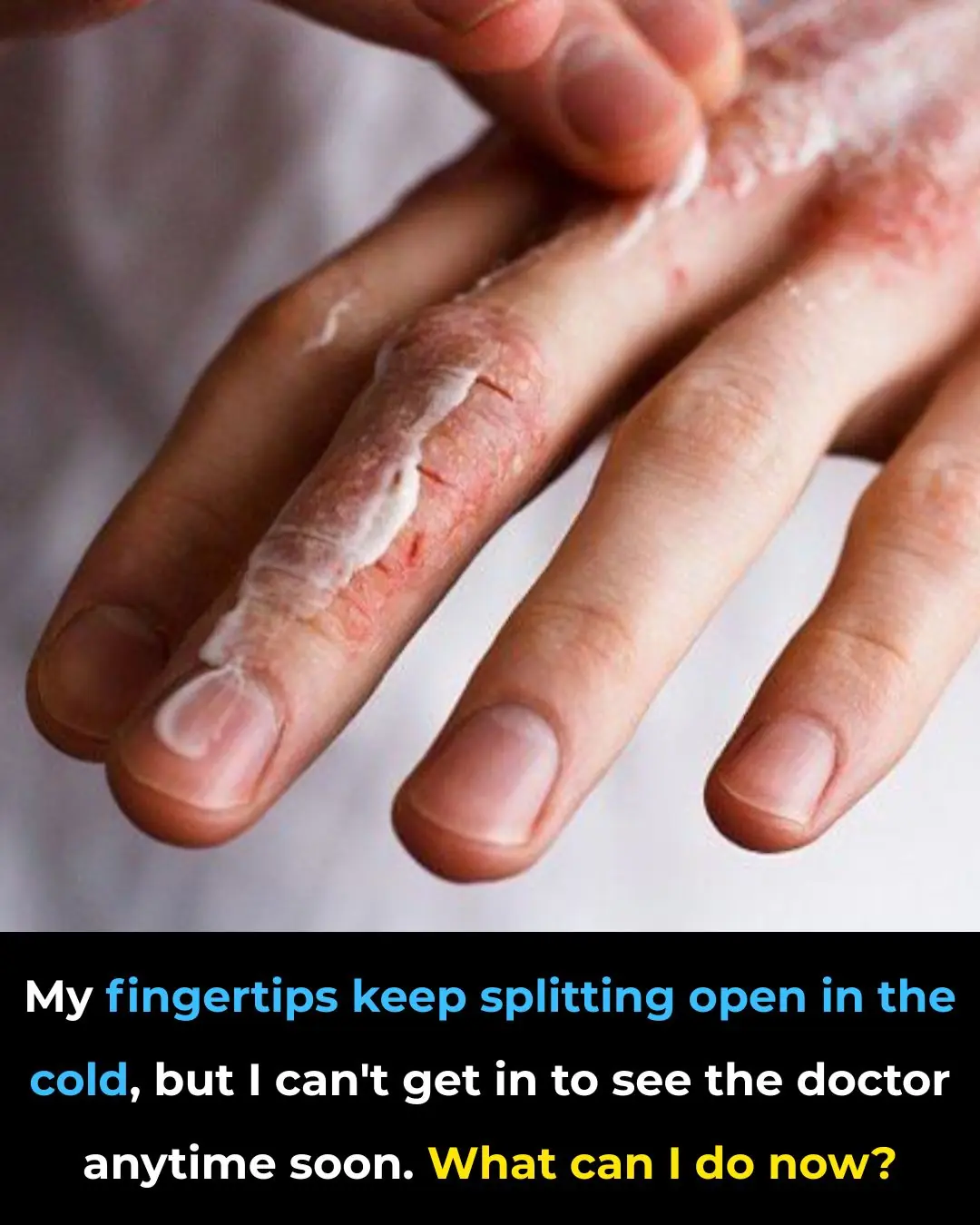
My fingertips keep splitting open in the cold, but I can’t get in to see the doctor anytime soon. What can I do now?

The Best Natural Gout Treatments: Remove Uric Acid Crystallization To Prevent Gout And Joint Pain

Quickly Drain You Lymph System Using Theses Simple Techniques to Boost Immunity and Remove Toxins

The Top 20 Essential Oils to Relieve Pain and Inflammation (Research Based)

Headache Above or Behind the Left Eye: Causes and Treatments

Root Canals May Lower Risk of Heart Disease, Diabetes
