FDA Approves Steam-Based Device for Prostate Cancer Treatment, but Questions Remain

The U.S. Food and Drug Administration (FDA) has approved a novel medical device designed to treat prostate cancer using water vapor, or steam, marking a new step in the evolution of minimally invasive cancer therapies. The device, known as the Vanquish Water Vapor Ablation System, was developed by Francis Medical, Inc. and aims to destroy prostate tumors through targeted thermal energy.
While the approval represents a significant technological milestone, experts caution that the clinical effectiveness of the device in fully eliminating prostate cancer has not yet been definitively established, according to the manufacturer itself.
What Is the Vanquish Water Vapor Ablation System?
The Vanquish system is a minimally invasive ablation device that delivers controlled bursts of high-temperature steam directly into prostate tissue. When the steam condenses inside the targeted area, it releases thermal energy that causes cell death, effectively destroying tumor tissue.
This approach builds on principles already used in other urologic treatments, such as steam-based therapies for benign prostatic hyperplasia (BPH), but applies the technology specifically to prostate cancer tumors.
Key features of the device include:
-
Precise delivery of water vapor to targeted prostate tissue
-
Short treatment times
-
Minimal incisions or invasive surgery
-
Potential for reduced recovery time compared with traditional treatments
Why Steam?
Steam ablation works by exploiting a basic physical principle: when steam condenses back into water, it releases a large amount of energy. This rapid energy transfer can cause localized tissue destruction without relying on radiation or freezing, as seen in other ablation techniques.
In theory, this allows clinicians to:
-
Target cancerous tissue while sparing surrounding structures
-
Reduce damage to nerves involved in urinary and sexual function
-
Offer a treatment option for patients seeking alternatives to surgery or radiation
FDA Approval: What It Does — and Does Not — Mean
FDA approval of the Vanquish system confirms that the device met regulatory standards for safety and technical performance. However, approval does not necessarily mean that the device has been proven to cure prostate cancer.
According to Francis Medical, Inc., the efficacy of the system in completely eliminating prostate cancer has yet to be fully established. This distinction is critical, as prostate cancer outcomes are typically measured over many years, with long-term data needed to assess recurrence rates, progression, and survival.
Current Evidence and Clinical Uncertainty
Early studies and feasibility trials suggest that steam ablation can successfully destroy targeted prostate tissue. However, several key questions remain unanswered:
-
Does the treatment reliably eliminate all cancer cells in the targeted area?
-
How does it compare to established treatments such as prostatectomy, radiation therapy, or focal therapies like HIFU and cryotherapy?
-
What are the long-term cancer control outcomes?
-
How often does cancer recur after treatment?
Until large-scale, long-term clinical trials are completed, these questions will remain open.
Potential Benefits for Patients
If future studies confirm its effectiveness, the Vanquish system could offer meaningful advantages for certain patients, particularly those with localized, low- to intermediate-risk prostate cancer.
Potential benefits may include:
-
Reduced treatment-related side effects
-
Lower risk of urinary incontinence
-
Less impact on erectile function
-
Shorter recovery and outpatient treatment
These potential advantages are especially appealing given the quality-of-life concerns that often accompany traditional prostate cancer treatments.
Risks and Limitations
As with any new medical technology, steam ablation is not without limitations. Possible concerns include:
-
Incomplete tumor destruction
-
Damage to nearby healthy tissue if not precisely targeted
-
Lack of long-term data on cancer control
-
Uncertainty regarding ideal patient selection
For now, experts emphasize that the technology should be considered investigational in terms of cancer cure, despite its regulatory approval.
What This Means for Prostate Cancer Care
Prostate cancer treatment is increasingly moving toward personalized and less invasive approaches, particularly for patients with early-stage disease. The approval of the Vanquish Water Vapor Ablation System reflects this broader trend and highlights ongoing innovation in urologic oncology.
However, clinicians and patients alike must balance innovation with evidence. Until more robust data are available, steam-based ablation should be viewed as a promising option under evaluation, rather than a proven replacement for established therapies.
Conclusion
The FDA approval of the Vanquish Water Vapor Ablation System represents an exciting technological development in prostate cancer treatment. By using steam to destroy tumor tissue, the device offers a novel, minimally invasive approach that could potentially reduce side effects and improve patient experience.
Yet, as the manufacturer acknowledges, its ability to definitively eliminate prostate cancer has not yet been proven. Ongoing clinical trials and long-term follow-up studies will be essential to determine whether this innovative technology can fulfill its promise.
For now, the device stands as a reminder that in medicine, approval is often the beginning of the story — not the final verdict.
News in the same category


Cannabis Hyperemesis Syndrome: Why Emergency Department Visits Surged During the Pandemic

Viral Infections Can Triple the Risk of Heart Attack and Stroke
Australian HPV Vaccination Marks Cervical Cancer Milestone
Vitamin K2 Supplementation and Vascular Health in Chronic Kidney Disease

Daily Prune Consumption and Bone Health in Postmenopausal Women

Montmorency Tart Cherry Juice as an Adjunctive Therapy in Ulcerative Colitis
Methylene Blue–Based Photodynamic Therapy as a Selective Strategy Against Breast Cancer Cells
Regenerating True Articular Cartilage: A Breakthrough in Joint Repair

Deuterium-Depleted Water and Long-Term Survival in Cancer Patients: Evidence from a Hungarian Population-Based Analysis

Daily cocoa extract supplements lowered cardiovascular disease deaths by 27%

Ivermectin stopped aggressive cancer cells from moving and spreading in lab tests
The Best Natural Gout Treatments: Remove Uric Acid Crystallization To Prevent Gout And Joint Pain
Quickly Drain You Lymph System Using Theses Simple Techniques to Boost Immunity and Remove Toxins
The Top 20 Essential Oils to Relieve Pain and Inflammation (Research Based)

Headache Above or Behind the Left Eye: Causes and Treatments
Root Canals May Lower Risk of Heart Disease, Diabetes

Mother-to-Infant Microbiome Transmission: Beyond Bacteria to Genes
Vitamin C Supplementation and Its Targeted Impact on the Human Gut Microbiome
News Post

Uterine Fibroids May Signal Increased Risk of Heart Disease, New Research Suggests

Cannabis Hyperemesis Syndrome: Why Emergency Department Visits Surged During the Pandemic

The secret button on a computer with ‘magical’ functions that not everyone knows about

Viral Infections Can Triple the Risk of Heart Attack and Stroke

Australian HPV Vaccination Marks Cervical Cancer Milestone

My outdoor faucet suddenly froze and now I’m seeing water seeping indoors — what should I do before a plumber can come?

Vitamin K2 Supplementation and Vascular Health in Chronic Kidney Disease

Daily Prune Consumption and Bone Health in Postmenopausal Women

Montmorency Tart Cherry Juice as an Adjunctive Therapy in Ulcerative Colitis

Methylene Blue–Based Photodynamic Therapy as a Selective Strategy Against Breast Cancer Cells

Regenerating True Articular Cartilage: A Breakthrough in Joint Repair

Deuterium-Depleted Water and Long-Term Survival in Cancer Patients: Evidence from a Hungarian Population-Based Analysis

Daily cocoa extract supplements lowered cardiovascular disease deaths by 27%

Ivermectin stopped aggressive cancer cells from moving and spreading in lab tests
These brown crusty spots keep showing up, and my doctor is booked for weeks given Christmas. Should I be concerned?
There’s this crusty little spot that keeps scabbing and reopening, and I can’t get in to see anyone yet. What could this be?

I had no clue about this
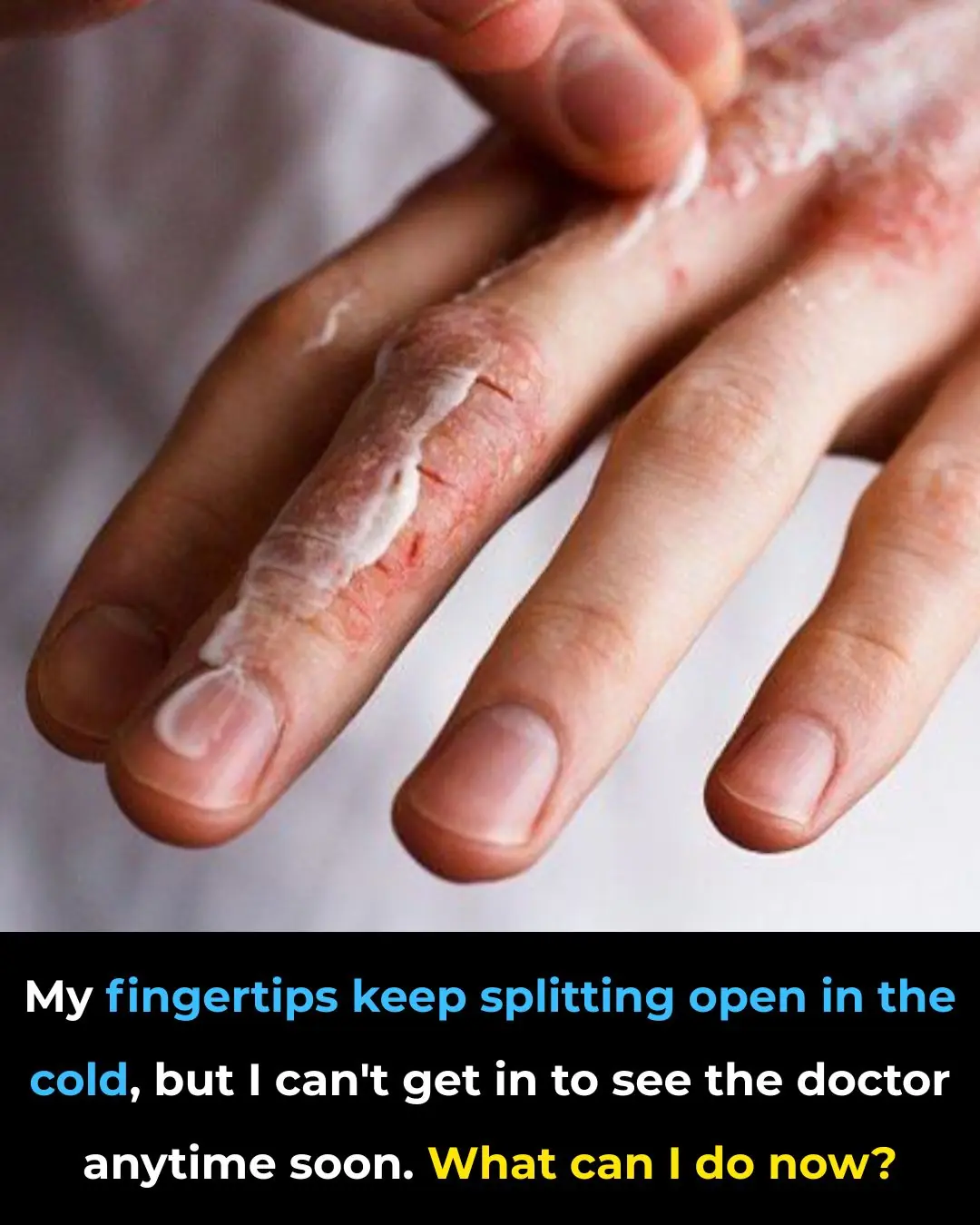
My fingertips keep splitting open in the cold, but I can’t get in to see the doctor anytime soon. What can I do now?
