
HMGB1 Identified as a Key Regulator of Aging and Tissue Regeneration
Scientists have uncovered a discovery that could fundamentally change how we understand aging and tissue repair. At the center of this breakthrough is a protein known as HMGB1 (High Mobility Group Box 1), which researchers now believe plays a pivotal role in driving chronic inflammation and age-related tissue damage. Remarkably, when scientists blocked the activity of this single protein in experimental models, damaged tissues began to repair themselves in ways that resemble youthful regeneration.
HMGB1 is normally involved in DNA organization and cellular signaling, but under conditions of stress, injury, or aging, it is released outside cells and acts as a powerful inflammatory signal. As people age, persistent release of HMGB1 contributes to chronic low-grade inflammation — often referred to as “inflammaging” — which interferes with normal healing, accelerates tissue degeneration, and impairs organ function. Researchers found that excessive HMGB1 effectively keeps the body locked in a constant state of damage response, preventing proper repair.
In recent studies, scientists observed that inhibiting HMGB1 signaling allowed tissues to exit this inflammatory state and re-enter regenerative pathways. Muscles, connective tissues, and organs showed improved structural repair, reduced scarring, and enhanced cellular renewal. Instead of merely slowing degeneration, the intervention appeared to restore the body’s own ability to heal itself, suggesting that aging may be driven less by irreversible damage and more by disrupted repair signals.
This finding challenges the long-standing view that aging is simply the accumulation of wear and tear. Instead, it supports the idea that aging is, at least in part, a regulatory failure — a condition in which the body retains the capacity to regenerate but is actively prevented from doing so by inflammatory signaling molecules like HMGB1. By targeting this protein, scientists may be able to shift tissues from a chronic damage state back into a repair-oriented mode.
The implications for medicine are profound. If these mechanisms translate safely to humans, therapies aimed at modulating HMGB1 could one day improve recovery from injury, slow age-related diseases, and enhance tissue regeneration without genetic modification. Such treatments could impact conditions ranging from muscle wasting and cardiovascular disease to neurodegeneration and organ fibrosis. Importantly, this approach focuses on restoring balance rather than forcing artificial regeneration, potentially reducing side effects.
Researchers caution that this work is still in experimental stages, and aging is a complex, multi-factorial process involving genetics, metabolism, immune function, and environmental factors. Blocking HMGB1 alone is unlikely to be a universal cure for aging. However, it represents a powerful proof of concept that aging processes may be reversible at the signaling level, not fixed at the structural level.
Leading research institutions and scientific journals have increasingly highlighted HMGB1 as a critical regulator of inflammation, tissue repair, and aging. Findings from the National Institutes of Health, Nature Aging, Cell, Science Translational Medicine, and The Journal of Clinical Investigation support the growing view that controlling inflammatory signaling pathways may unlock new strategies for regenerative medicine and healthy longevity.
This is not science fiction, but it is still science in progress. Even so, the discovery reframes aging from an inevitable decline into a biological state that may be adjustable, treatable, and partially reversible. Rather than merely extending lifespan, future therapies inspired by this research may focus on extending healthspan — the years of life spent in good physical and cognitive health.
Bottom line: HMGB1 may act as a master switch that suppresses the body’s natural repair systems during aging. Blocking this protein has revealed the potential to restore regenerative capacity, opening a new frontier in aging and regenerative medicine.
News in the same category


Remission Is a Reminder of the Power of Belief
A Scientist Injected Herself With Viruses to Treat Cancer — and It Worked

Forget Plaque: Alzheimer’s May Be a Brain Energy Crisis
Regenerative Ear Drop Therapy Restores Natural Hearing in Early Trials

ADHD Treatment in Childhood Linked to Higher Adult BMI and Slight Height Reduction

What It Really Means When Your Partner Starts Kissing You With Their Tongue More Often
Why Neck Skin Sags as We Age

Doctors Reveal: Eating Cabbage Can Trigger Hidden Health Problems If You’re Not Careful

The Winged Bean Secret: A Simple Vegetable With Big Benefits for Eyes, Immunity, and Heart Health
Why Do I Get Skin Tags on My Neck or Armpits—and How Can I Get Rid of Them? Experts Explain

Doctors Reveal What Eating Broccoli Really Causes in the Body

What Weak or Brittle Nails May Be Telling You About Your Health
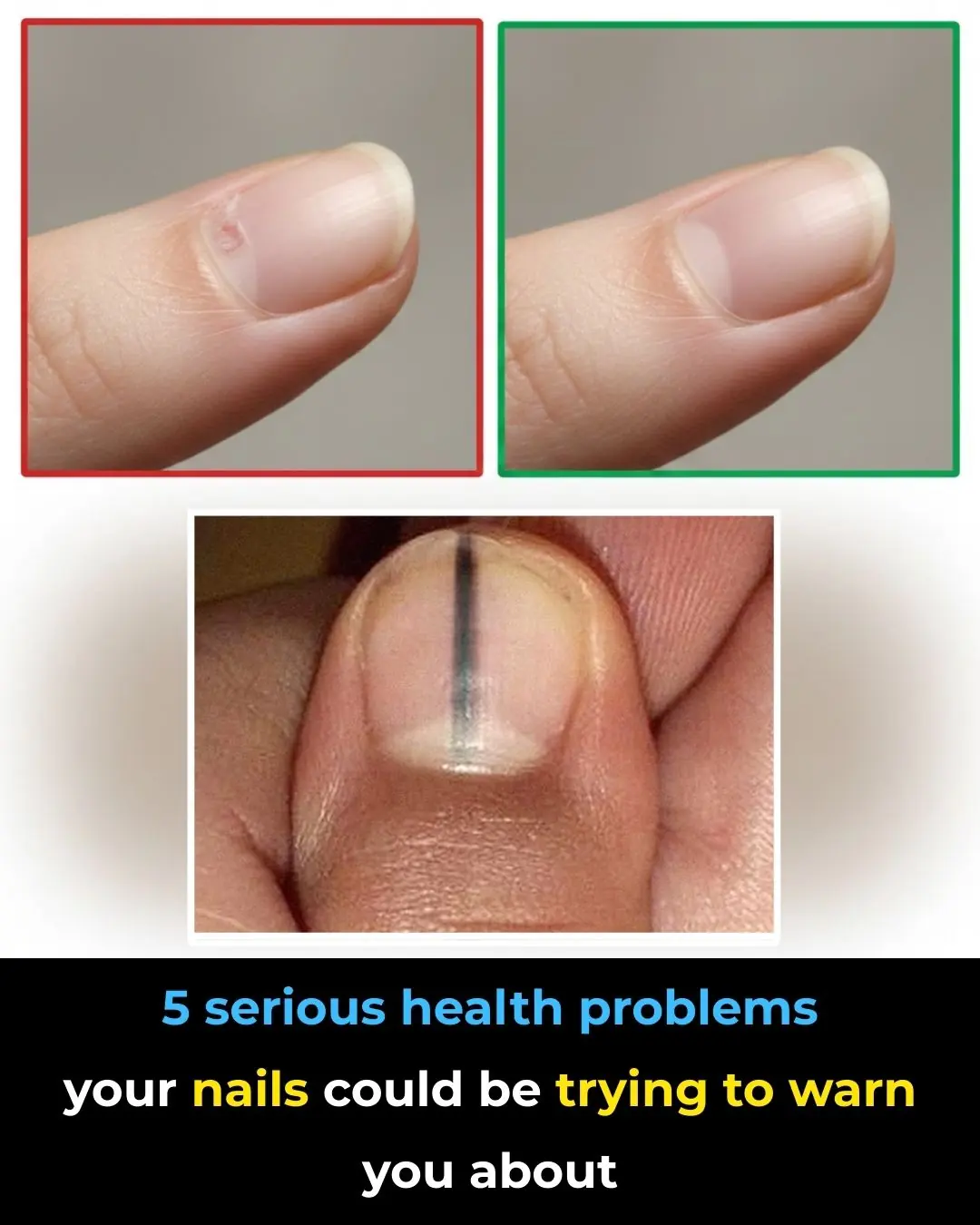
Nail Clues: 5 Health Problems You Shouldn’t Ignore
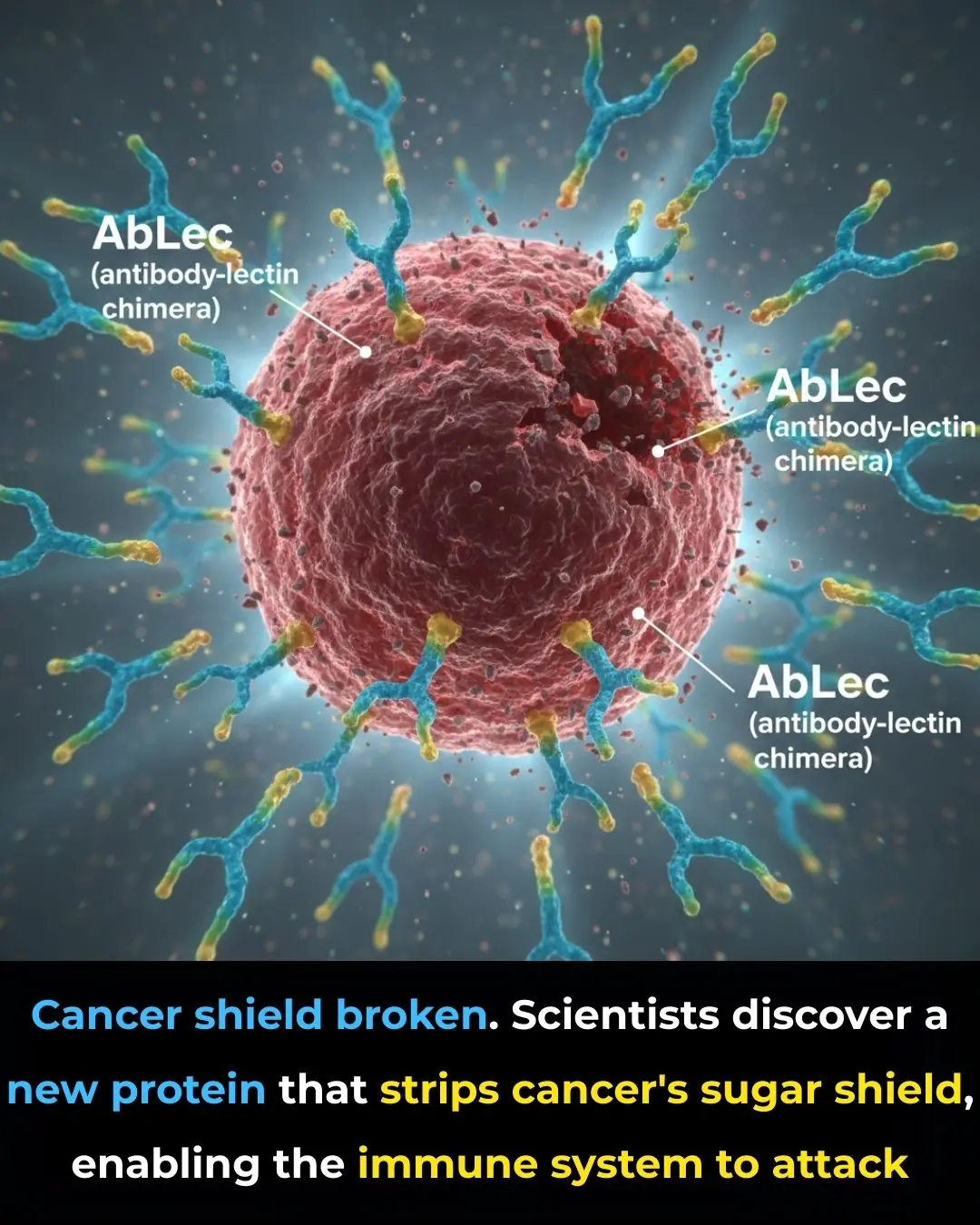
New Protein Breakthrough Unmasks Cancer Cells, Boosting the Power of Immunotherapy

Sugary Soft Drinks, Gut Bacteria, and Depression: How What You Drink May Shape Mental Health

Are You Using New Tools to Support Patients With DD-CKD Anemia?

Drug Shortages in the United States Are Forcing Major Changes in Primary Care Practice

Five Reasons to Eat One Banana a Day: A Simple Habit for Better Health and Longevity
News Post

When frying pork fat, some people add salt, others add water, but chefs use pure white fat that lasts a long time without spoiling.

Restoring Brain Energy Reverses Advanced Alzheimer’s Pathology in Preclinical Models

Remission Is a Reminder of the Power of Belief

A Scientist Injected Herself With Viruses to Treat Cancer — and It Worked

Forget Plaque: Alzheimer’s May Be a Brain Energy Crisis

Regenerative Ear Drop Therapy Restores Natural Hearing in Early Trials

Department of Education says nursing is no longer a professional degree. See how it affects student loans

Rep. Sheila Cherfilus-McCormick of Florida charged with stealing FEMA money, using it for her campaign, DOJ says

GOP Rep. Marjorie Taylor Greene will leave Congress after five turbulent years

Jimmy Cliff, reggae legend and Jamaican icon, dies at 81

MMA Icon Turned Hollywood Star Honored With Bruce Lee Award After 78 Movies

Ice-T Cites Law and Order: SVU’s ‘Budget’ Constraints as the Reason Fin Has Been MIA This Season

The Manager Threw Her Last Meal In The Trash, Not Realizing The “Beggar” Watching Owned The Entire Mall

ADHD Treatment in Childhood Linked to Higher Adult BMI and Slight Height Reduction

What It Really Means When Your Partner Starts Kissing You With Their Tongue More Often

Easy Tips to Keep Your Bathroom Smelling Fresh All Day

Why Neck Skin Sags as We Age

Doctors Reveal: Eating Cabbage Can Trigger Hidden Health Problems If You’re Not Careful
