
Thymoquinone and Breast Cancer Cell Suppression: Evidence from Preclinical Research
Breast cancer remains one of the most prevalent malignancies worldwide, and despite major advances in early detection and treatment, resistance to therapy and disease recurrence continue to pose significant challenges. For this reason, there is sustained scientific interest in bioactive natural compounds that may complement existing treatments or inspire new therapeutic strategies. One such compound is thymoquinone (TQ), the main bioactive constituent of Nigella sativa (black seed). A comprehensive 2019 review published in Biomedicine & Pharmacotherapy summarizes a substantial body of laboratory research demonstrating that thymoquinone exerts potent anti-cancer effects against human breast cancer cells under controlled experimental conditions.
According to the studies reviewed, thymoquinone significantly reduced the viability of several well-established human breast cancer cell lines, most notably MCF-7 and T47D cells. These cell lines are widely used models representing hormone-responsive breast cancer and provide a standardized platform for evaluating anti-proliferative compounds. When cancer cells were exposed to thymoquinone at concentrations ranging from 25 to 100 μM for periods of 24 to 72 hours, researchers consistently observed strong growth inhibition. This reduction in cell viability was not merely due to nonspecific toxicity but was associated with well-characterized biological processes, including cell-cycle arrest and the activation of programmed cell death pathways.
One of the key mechanisms identified was apoptosis, a tightly regulated form of cell death that is often defective in cancer cells. Thymoquinone treatment activated multiple apoptotic signaling pathways, including the activation of caspases—enzymes that play a central role in dismantling cancer cells during apoptosis. In parallel, thymoquinone increased the production of reactive oxygen species (ROS) within cancer cells. While moderate ROS levels are part of normal cellular signaling, excessive ROS can overwhelm antioxidant defenses, damage cellular components, and trigger apoptosis. Cancer cells, which already operate under elevated oxidative stress, appear particularly vulnerable to this effect.
At higher concentrations and longer exposure times, some experiments reported exceptionally high levels of cancer cell death, ranging from approximately 70% to 90%. These findings indicate a strong dose- and time-dependent response, a hallmark of biologically meaningful anti-cancer activity in vitro. In addition to inducing apoptosis, thymoquinone was shown to cause cell-cycle arrest, preventing cancer cells from progressing through critical checkpoints required for division. By halting proliferation and simultaneously activating death pathways, thymoquinone effectively attacked breast cancer cells on multiple fronts.
The review also notes that, in some cases, these in-vitro findings were supported by animal tumor models, where thymoquinone demonstrated tumor-suppressive effects consistent with those observed in cell culture. Such in vivo data, while still preclinical, provide an important bridge between isolated laboratory experiments and potential future clinical applications. Nevertheless, the authors emphasize that the majority of evidence remains rooted in controlled laboratory settings.
Importantly, the review is explicit about the limitations of the existing research. The concentrations of thymoquinone used in cell culture experiments may not be easily achievable in human tissues through diet or supplementation, and the behavior of isolated cancer cells does not fully reflect the complexity of tumors in the human body. Factors such as immune responses, metabolism, tumor microenvironment, and drug distribution can substantially alter therapeutic outcomes. As a result, the findings do not demonstrate that thymoquinone can treat breast cancer in humans.
In conclusion, laboratory studies summarized in the 2019 Biomedicine & Pharmacotherapy review provide strong evidence that thymoquinone can markedly reduce the viability of human breast cancer cells under experimental conditions. Through mechanisms involving oxidative stress, cell-cycle arrest, and apoptosis, thymoquinone achieved up to 70–90% cancer cell death in some models (Biomedicine & Pharmacotherapy, 2019). While these results highlight thymoquinone as a promising anti-cancer compound at the preclinical level, they also underscore the need for further animal studies and carefully designed human trials before any clinical relevance can be established.
News in the same category


Ginger Supplementation and Cardiovascular Inflammation: Evidence from a Double-Blind Randomized Clinical Trial
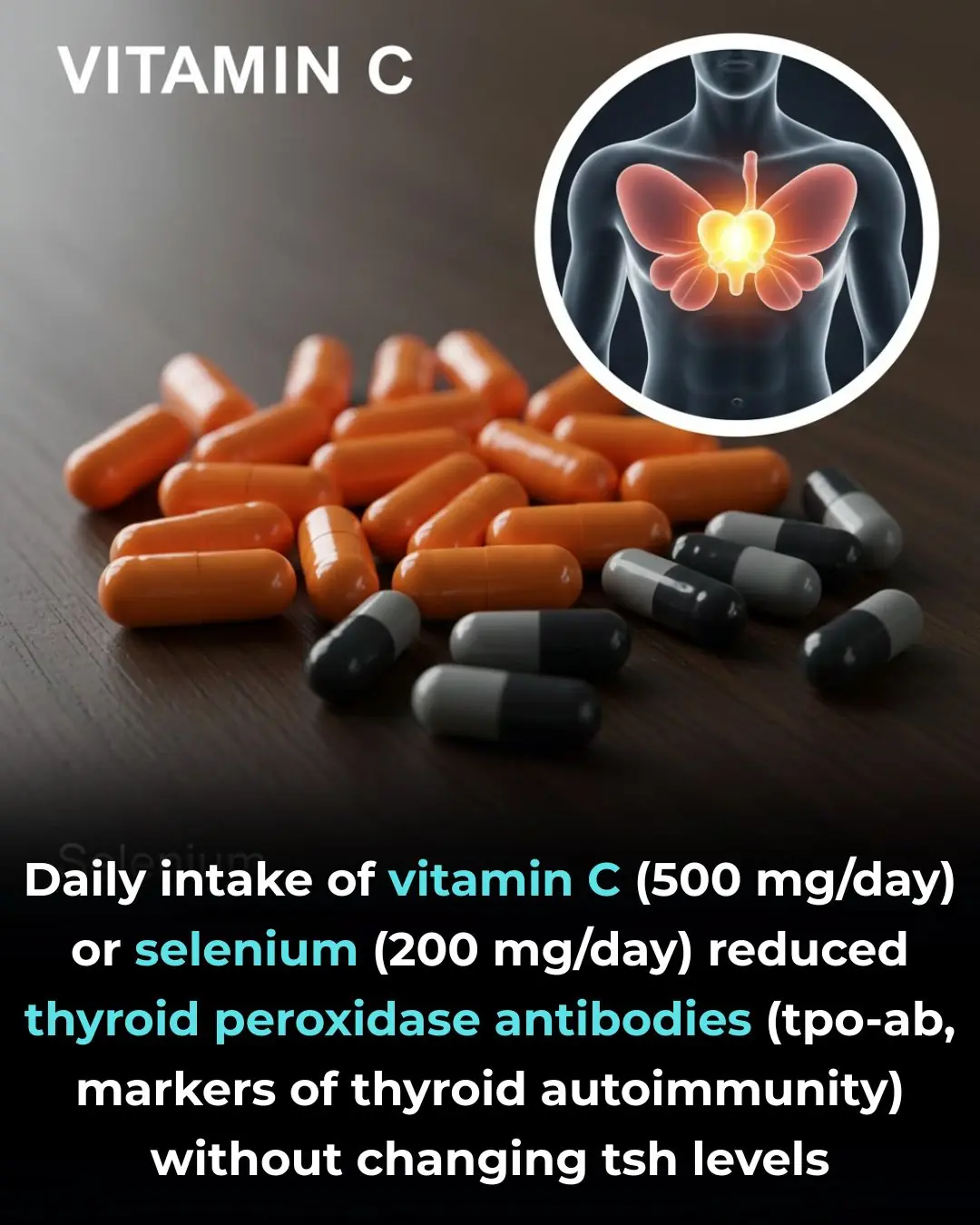
Antioxidant Supplementation and Thyroid Autoimmunity: Evidence from a Randomized Controlled Trial

Chios Mastic Gum as an Anti-Inflammatory Intervention in Crohn’s Disease and Vascular Inflammation

Garlic Supplementation and Metabolic Improvement in Non-Alcoholic Fatty Liver Disease
Potassium Deficiency – Causes, Symptoms and What To Do
14 Warning Signs of Low Magnesium Levels and What to Do About It (Science Based)

Proven Health Benefits of Beets and Fermented Beets (Science Based)

3 Reasons Onions Might Upset Your Stomach
MRI vs PET: Which Imaging Modality Better Detects Prostate Cancer Recurrence?

Hidden Spread of Pseudomonas aeruginosa From Lung to Gut in Hospitalized Patients
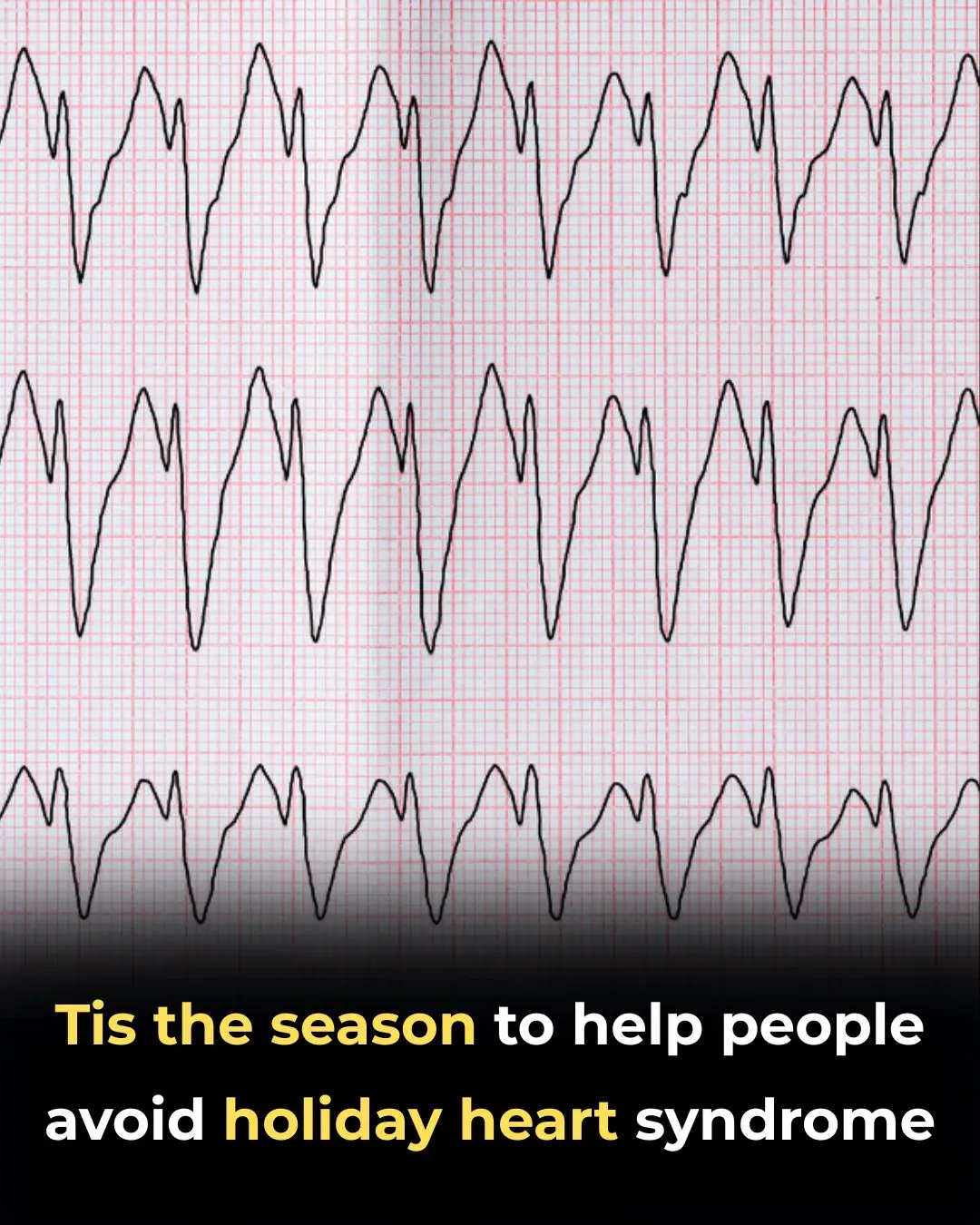
‘Tis the Season to Help People Avoid Holiday Heart Syndrome
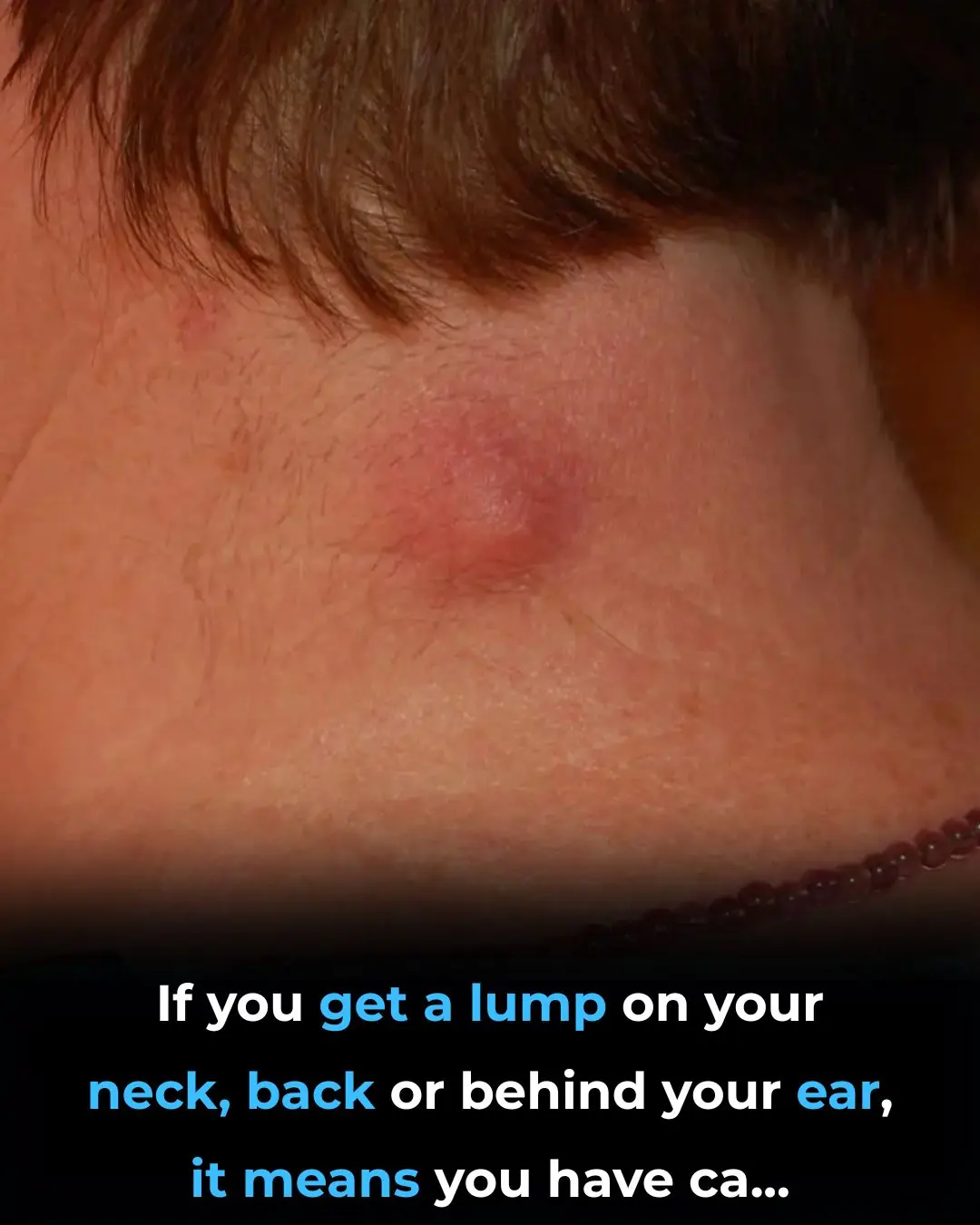
Got a lump on your neck, back or behind your ear? This is what you need to know

What Your Skin Could Be Telling You About Hidden Health Issues

How to Choose Fresh and Delicious Pork: Should You Pick Lighter or Darker Pieces?
How to Choose Fresh and Delicious Pork: Should You Pick Lighter or Darker Pieces?

4 Types of Vegetables Most Effective in Preventing Cancer, According to Doctors: Eating Them Regularly Is Great for Your Health

Stroke and Cerebral Infarction Prevention: Remember These 3 Indicators, 1 Disease, and 6 Key Habits

Optimism as a Psychosocial Predictor of Exceptional Longevity

Why Ages 36–46 Matter: Midlife as a Critical Window for Long-Term Health
News Post

Bee Propolis and Infertility in Endometriosis: Evidence from a Pilot Randomized Clinical Trial

Ginger Supplementation and Cardiovascular Inflammation: Evidence from a Double-Blind Randomized Clinical Trial

Antioxidant Supplementation and Thyroid Autoimmunity: Evidence from a Randomized Controlled Trial

Chios Mastic Gum as an Anti-Inflammatory Intervention in Crohn’s Disease and Vascular Inflammation

Garlic Supplementation and Metabolic Improvement in Non-Alcoholic Fatty Liver Disease

If you drink cucumber water every morning, this is what happens to your body

I soaked my feet in apple cider vinegar. 15 mins later, this is what happened

I need this ‘Liquid Gold.’

Potassium Deficiency – Causes, Symptoms and What To Do

14 Warning Signs of Low Magnesium Levels and What to Do About It (Science Based)

Proven Health Benefits of Beets and Fermented Beets (Science Based)

Putting this in the vase box not only helps protect the chrysanthemums but also makes the vase more delicious

How to preserve cilantro so it stays fresh, green, and fragrant for a whole month

3 Reasons Onions Might Upset Your Stomach

Vaseline Uses and Benefits for Skin, Lips and Hair | Petroleum Jelly Benefits

Beetroot Face Gel for Clear Skin – Rosy Cheeks & Pink Blushing Skin

How i use CUCUMBER for Skin & Eyes : Remove Dark Circles & Get Glowing Skin

Tips for removing grease from an air fryer
